Open Journal of Hepatology
Alcohol, hepatitis C screening and hepatic fibrosis in drug users
Di Nino F1*, Chaffraix F1,2, Schaeffer M3 and Doffoel M11
2Association SOS Hépatites Alsace-Lorraine, Strasbourg, France
3Pôle de Santé Publique, Secteur méthodologie et biostatistiques, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Cite this as
Di Nino F, Chaffraix F, Schaeffer M, Doffoel M (2019) Alcohol, hepatitis C screening and hepatic fibrosis in drug users. Open J Hepatol 1(1): 004-009. DOI: 10.17352/ojh.000002Background: In drug users, viral hepatitis C, alcohol abuse, and drug use are three interconnected public health challenges.
Methods: This study assessed the impact of alcohol on hepatitis C screening and hepatic fibrosis in this patient population. In total, 934 substance users were included and divided into three groups: Group A, alcohol use disorder only (n = 511); Group ISDU, intravenous and snorting drug users (n = 142); Group ISDAU, intravenous and snorting drug users with alcohol use disorder (n = 281). A FibroScan was performed first, after which participants were proposed to undergo screening for HCV.
Results: The HCV screening rate was significantly lower in Group A (62%) than in the ISDAU or ISDU groups (81% and 85% respectively) (p<0.001). The rate of HCV seropositivity was lower in Group A (4.4%), whereas it was significantly higher in the ISDAU group than ISDU group (42.3% vs 30.0%, p = 0.02). The rates of significant fibrosis and severe fibrosis were higher in Group A (34% and 21%) and the ISDAU group (29% and 18%) than in ISDU (15% and 7%) (p<0.001). While entering, in addition to the group, age, gender, smoking status (cannabis and tobacco), drug consummation, HCV seropositivity, and BMI as co-variables in a multivariate model, only age correlated with fibrosis (p<0.001). Considering age, there was no difference in impact among the different substances on the fibrosis score.
Conclusion: Alcohol consumption impacts the health status of drug users. It is thus appropriate to early identify alcohol consumption in drug users and consider alcohol as a risk factor for severe fibrosis and HCV transmission. Alcohol consumption warrants the strengthening of HCV screening and hepatic fibrosis assessment.
Abbreviations
HCV: Hepatitis C Virus; ISDU: Intravenous and Snorting Drug Users; ISDAU: ISDU with Alcohol use Disorder; CSAPA: Centres de Soins, d’Accompagnement et de Prévention en Addictologie; Care and Prevention Addiction Centers; RHR: Risk and Harm Reduction; SELHVA: Service Expert de Lutte Contre Les Hépatites Virales d’Alsace; DSM-5: Diagnostic and Statistical Manual of Mental Disorders. 5th Ed; CMUc: Couverture Maladie Universelle Complémentaire (free supplementary universal health insurance); BMI: Body Mass Index; DAA: Direct-Acting Antivirals
Introduction
Viral hepatitis C, along with alcohol use disorder and intravenous or snorting drug use, are three intrinsically linked public health challenges in France [1-8]. Intravenous and snorting drug users (ISDUs) represent the largest population responsible for hepatitis C virus (HCV) transmission in France.
Alcohol consumption is known to induce the progression of hepatic fibrosis in chronic hepatitis C patients [2-4,6,7]. Today, assessing hepatic fibrosis has become much simpler using non-invasive methods such as transient elastography [9].
In France, the front-line care for ISDUs is offered at support, care, and prevention addiction centers (Centres de Soins, d’Accompagnement et de Prévention en Addictologie or CSAPA), which were created in 2008 based on the merging of specialized drug addiction clinics and alcohol abuse outpatient centers [10,11]. Therefore, the CSAPAs also treat individuals suffering from alcohol use disorders. As social healthcare institution, these centers have the advantage of being well-placed in communities, offering multidisciplinary care, while ensuring long-term treatment and follow-up [10]. The risk and harm reduction (RHR) strategy employed at the CSAPA encompasses all the measures aimed at reducing hepatotropic virus contaminations and alcohol consumption.
The primary goal of this study, conducted as part of a novel training and research program, was to evaluate the impact of alcohol consumption on hepatitis C screening in ISDUs followed-up in CSAPAs. The secondary objectives were to investigate the impact of alcohol consumption on hepatic fibrosis severity and determine whether this factor should be considered an indicator of severity and, thus, require the implementation of enhanced risk and harm reduction (RHR) strategies in ISDUs.
Patients and Methods
This study involved substance users followed-up in seven different CSAPAs in the Alsatian territory, France (five hospital-based and two city-center associations). The study was conducted as part of the training and research program launched in 2012 by the Expert Service to Combat Viral Hepatitis in Alsace (Service Expert de Lutte contre les Hépatites Virales d’Alsace [SELHVA]). This program, supported by the regional healthcare agency and SOS hepatitis association of the Alsace-Lorraine region (SOS Hépatites Alsace-Lorraine), was designed to initiate a coordinated strategy to reduce the risks of HCV transmission among drug users followed-up in CSAPAs. Within this program, a theoretical, multidisciplinary training scheme was proposed to healthcare professionals (doctors and nurses) and social or educational professionals (psychologists, social workers, etc.), in the form of workshops and practical training for assessing hepatic fibrosis using transient elastography with mobile FibroScan® (Echosens, Paris, France). This course took place in each CSAPA. The FibroScan® was made available to each center following a schedule outlined by the SELHVA.
The inclusion period was from December 2012 to March 2016. Doctors proposed participation to the study to all substance users presenting at a CSAPA during the period when the Fibroscan® was available. The physicians provided an information sheet concerning the study to all substance users upon entering the program. Once fully informed about the study, the substance users were free to choose whether or not to participate. Those accepting the invitation to enter the study completed and signed an informed consent form. The program comprised a hepatitis C screening offered to all participants, followed by a mobile FibroScan® evaluation. The FibroScan® is a non-invasive method for evaluating the severity of chronic liver disease by accurately detecting the stage of fibrosis on a scale from 0 to 75KPa, corresponding to a fibrosis stage ranging from F0 to F4 according to the METAVIR classification [12,13]. Stages ≥F2 (over 7.5Kpa) signifies significant fibrosis. The fibrosis is considered severe for Stages F3 and F4 (over 9.5Kpa) with Stage F4 corresponding to cirrhosis.
Screening for hepatitis C (anti-HCV antibodies) was performed on a vein or capillary blood sample using classic immunoenzymatic assays. For hospital-based CSAPA, the samples were taken at the center, whereas for CSAPA associations, the samples were taken at the closest medical analysis laboratory. Patients with positive HBs Ag or HIV serology were excluded from the study.
All clinical data were collected by the physicians of each center and entered into a protected and anonymous electronic record. Substance use and psychiatric disorders were additionally recorded by the physicians and classified according to the DSM-5 [14].
Substance users were categorized into three groups depending on their principal addiction based on the DSM5: Group A, alcohol use disorder; Group ISDU, intravenous and snorting drug users; Group ISDAU: ISDUs with alcohol use disorder.
The results between the groups were compared by means of standard position and dispersion statistical analyses. For comparisons of quantitative variables among several subgroups, such as Screened 0/1, analysis of variance or the Kruskal-Wallis test were employed. A multivariate analysis of the risk factors of the variable of interest (continuous score) was performed using a gamma regression model. All analyses were performed using software R Version 3.1.
Results
In total, 961 patients were recruited, among whom four refused participating to the study protocol (0.4%). FibroScan failure was observed in 11 patients (1.1%). For 12 other cases, the FibroScan results proved un-interpretable because of obesity issues (1.2%). As a result, 934 patients were evaluated.
Group A comprised 511 users, Group ISDU 142, and Group ISDAU 281. The main demographic and addiction characteristics of all three study groups are presented in table 1. Compared to the ISDU and ISDAU groups, substance users were more prevalent in Group A, they were older, consumed less tobacco and cannabis, and were less covered by free supplementary universal health insurance (couverture maladie universelle complémentaire [CMUc]), an indicator of poverty in France [15]. The ISDU and ISDAU groups were comparable in terms of age, frequency of tobacco and cannabis use, and CMUCs insurance cover.
The frequency of psychiatric comorbidities and BMI values were comparable across all three groups.
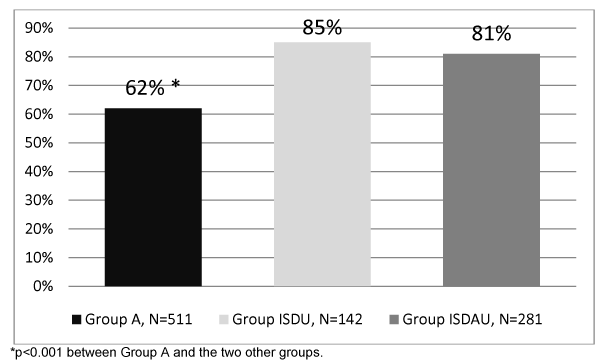
The rate of hepatitis C screening was significantly lower in Group A (62%) compared to the ISDU and ISDAU groups (85% and 81%, respectively [p <0.001]) (Figure 1).
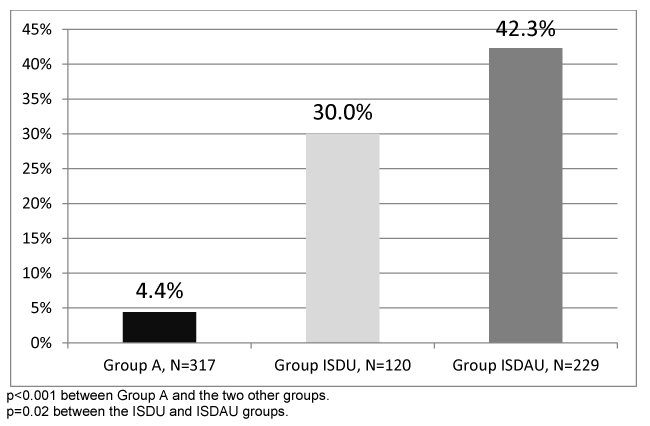
The rate of positive HCV tests was 4.4% in Group A, significantly lower (p <0.001) than that of the ISDU (30%) and ISDAU (42.3%) groups. This rate was also recognizably higher in the ISDAU than ISDU group (p=0.02) (Figure 2).
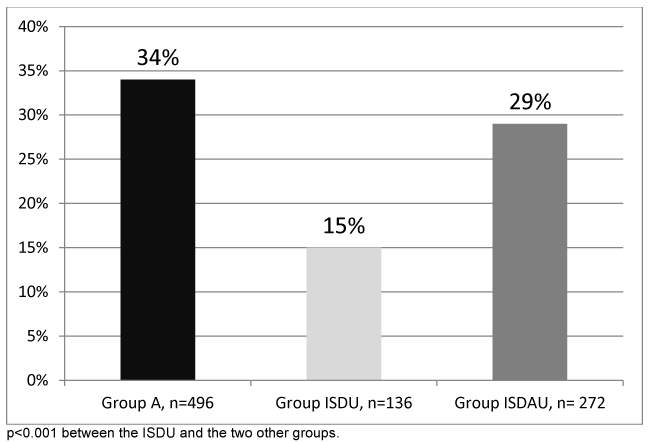
The rate of patients agreeing to undergo FibroScan® was 99.6%. The distribution of fibrosis stages is presented in figure 3. The rates of significant fibrosis (F ≥2) and severe fibrosis (F3-F4) were higher in Group A and the ISDAU group (34% and 29%, 21% and 18% respectively) than in the ISDU group (15% and 7%) (p<0.001). There was no significant difference between Group A and the ISDAU group. Comparisons of the fibrosis score among the three groups, using the Gamma regression model, revealed that the groups did not have an equivalent fibrosis score (p=0.0016). More precisely, the ISDU group presented a significantly lower score than Group A (coefficient in the model -0.42+-0.11, p=0.0002). When considering the group, in addition to gender, age, smoking status (cannabis and tobacco), drug consummation, HCV seropositivity and BMI, as covariables in the multivariate model, only one covariable correlated to the fibrosis score, namely the age of the patient (coefficient in the model 0.02+-0.0037, p<0.001).
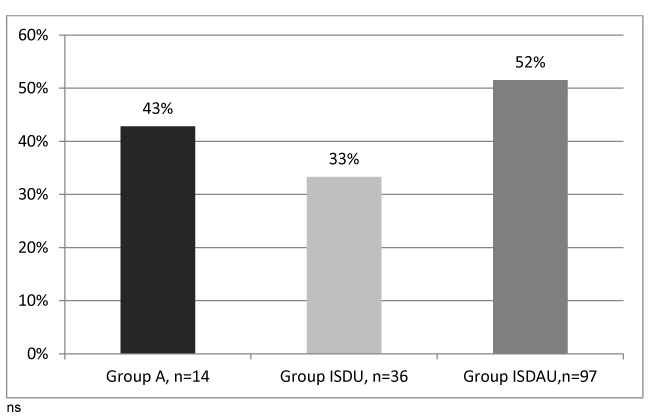
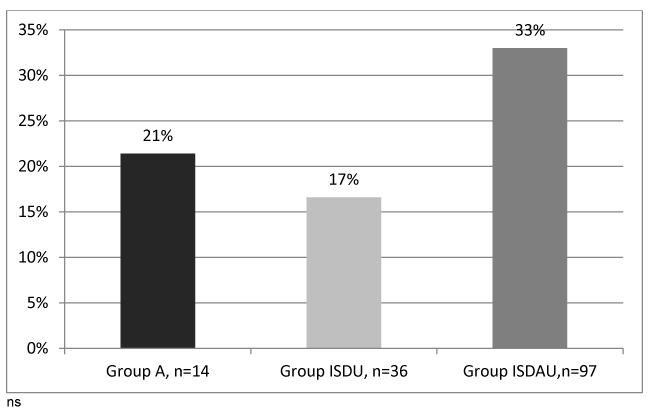
The distribution of fibrosis stages in HCV positive patients in the three groups is presented in figure 4. The rates of significant fibrosis (F ≥2) and severe fibrosis (F3-F4) were appears higher in ISDAU group (52% and 33% respectively) than in the A group (43% and 21%) and in the ISDU group (33% and 17%), but there was no significant difference between the three groups probably related to a lack of statistical power due to low numbers.
Discussion
The Fibroscan® program implementation enabled centers to promote awareness and action among CSAPA professionals concerning not only viral hepatitis but also different liver diseases, notably linked to alcohol consumption. Systematic screening in the alcohol-abuse group thus appears justified, particularly for HCV, given that the rate of HCV seropositivity proved to be four-times higher in this group than the general population [16], probably due to a background of high-risk behavior in these individuals. Moreover, 21% of drug users who also suffer from alcohol addiction had CMUc insurance cover, a precariousness indicator, while 10% presented a psychiatric comorbidity, both being considered as risk factors for HCV [17].
In our study, HCV screening was performed using vein or capillary samples, which proved highly effective for drug users, with seropositivity rates surpassing 80%. This screening adhered to current recommendations [17], given that the ISDUs constitute the main reservoir for HCV infection, as well as the first to be affected by new infections. It is all the more justified that direct-acting antivirals (DAAs) are administered, irrespective of fibrosis stage [18], as they are highly effective and well tolerated. Additionally, CSAPAs help break down barriers regarding healthcare supply [19], as that these centers, on the front lines of substance abuse, are often visited by ISDUs for several different reasons, be it healthcare, social care, or RHR. Alternative HCV screening tests based on venous blood samples, such as microsampling, saliva swab, dried blood spot, and rapid diagnostic tests [20,21], should likewise be instrumental in optimizing screening and rendering it more systematic. In our study, 30% of the non-alcohol-abusing ISDUs were HCV-positive, versus 42% of ISDUs with alcohol-abuse disorders (p=0.02). These rates are lower than those found in the RECAP/CSAPA French national survey, which reported 45.2% of all drug users testing positive in 2015. However, in the RECAP/CSAPA survey [22], reported data were only declarative, whereas the last few years have seen a decreasing trend in declared HCV prevalence [23]. Also, a quarter of drug users are not aware of their viral status [24]. The impact of alcohol consumption on HCV seroprevalence has not previously been studied in drug users attending CSAPAs. The increase in HCV seropositivity rates in correlation with alcohol consumption could be related to an increase in behaviors posing high risk of viral transmission. In our study, psychiatric comorbidities were more common when drug users were afflicted by alcohol addiction (ISDAU), though the difference was not statistically significant. It is thus clear that RHR measures should be reinforced for drug users with concomitant alcohol addiction.
In patients with chronic HCV infection, chronic alcohol consumption induces more rapid progression of fibrosis [17]. This harmful impact of alcohol was observed for a consumption exceeding 40g/day, with a higher prevalence of cirrhosis in excessive drinkers than teetotalers: 38% vs 18% [17]. In our study, mean alcohol consumption proved to be very high in drug users, surpassing 80g/day. Furthermore, nearly half of drug users were reported to consume cannabis, a substance known, like age, to correlate with accelerated fibrosis progression in chronic hepatitis C [25]. The high prevalence of severe fibrosis at Stage F3-F4 we found in drug users suffering from alcohol disorders, nearing 20%, was, therefore, hardly surprising. On the other hand, the rate of severe fibrosis observed proved to be lower in the ISDU (7%) than ISDAU (18%) group, whereas their age and frequency of cannabis use did not significantly differ. Likewise, the rate of severe fibrosis observed was lower in the ISDU (7%) than in Group A (18%), but with a significant difference in age and cannabis use. Thus, among drug users without alcohol consumption, the progression of fibrosis proved to be minor (p <0.01). Overall, these results demonstrate that alcohol plays a major role in fibrosis progression in drug users. The rate of severe fibrosis was, in fact, similar in the groups of alcohol consumers who did not use drugs (21%). The impact of the fibrosis score was assessed using multivariate analyses. Overall, the fibrosis score depended solely on age (p <0.001), regardless of gender, HCV seropositivity, BMI, whether or not there was drug use, in association or not with alcohol, tobacco, or cannabis consumption. When adjusting for age, there was no difference in impact on the fibrosis score among the different substances.
Assessing fibrosis using Fibroscan® was found to present several limitations in our study. The assessments were performed by several different operators at the CSAPA, either general practitioners or nurses. Nevertheless, it must be mentioned that the teams had followed the same theoretical and practical training, provided by the Fibroscan® program experts. In addition, the elasticity values produced were all interpreted by the same hepatology specialist of this expert team, who considered the medical record data of the drug users and the quality of measuring procedures (number of valid measurements, dispersion of data, etc.). However, the threshold elasticity values considered for the different fibrosis stages were those pertaining to hepatitis C rather than alcohol liver disease [26], given that our study was focused on detecting viral hepatitis conditions, and, in particular, hepatitis C. It must, nonetheless, be noted that the threshold values for the F3-F4 stages (9.5kPa) proved to be quite similar between hepatitis C and alcohol liver disease [27].
In the future, it will probably be justified to combine Fibroscan® with another biological screening test [28] to more accurately evaluate the fibrosis stage. Nevertheless, in our study, Fibroscan® proved to be an effective means for hepatitis C screening. The rate of user acceptance to undergo this examination was almost 100%. Receiving Fibroscan® results enabled substance users to gain real awareness of their hepatic disease. It was used across all CSAPAs, unifying the different teams, and thus constitutes a highly-useful tool for RHR strategies.
Conclusions
In conclusion, in drug users followed-up at CSAPAs, alcohol-use disorders correlate with higher risk of HCV seropositivity and severe fibrosis. Assessing and considering this disorder must thus be integrated and optimized among RHR and care strategies for drug users.
The lead author would like to acknowledge:
Ernwein F (1), Oster F (2), Bernard-Henri C (3), Kowalczyk J (3), Gaugler E (4), Huber N (5), Bonomi O (5), Bonnewitz ML (6), Pfeiffer C (7), Hoth A (8) and all the teams from each of the centers. Our thanks go also to Dr Gabrielle Cremer for writing assistance.
1. Service Expert de Lutte contre les Hépatites Virales d’Alsace (SELHVA), Hôpitaux Universitaires de Strasbourg, Strasbourg, France
2. Csapa Ithaque, Strasbourg, France
3. Csapa ALT, Strasbourg, France
4. Csapa, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
5. Csapa, Centre Hospitalier de Sélestat, Sélestat, France
6. Csapa, Centre Hospitalier Sainte-Catherine, Saverne, France
7. Csapa, Centre Hospitalier de Haguenau, Haguenau, France
8. Csapa, Centre Hospitalier de Wissembourg, Wissembourg, France
- Bellentani S, Tiribelli C (2001) The spectrum of liver disease in the general population: lesson from the dionysos study. J Hepatol 35: 531-537. Link: https://tinyurl.com/y3gqqoph
- Chen JD, Yang HI, Iloeje UH,You SL, Lu SN, Wang LY, et al. (2010) Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver related death. Gastroenterology 138: 1747-1754. Link: https://tinyurl.com/yysnlxlb
- Donato F, Tagger A, Chiesa R, Ribero ML, Tomasoni V, et al. (1997) Hepatitis B and C virus infection,alcohol drinking, cigarette smoking and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC study. Hepatology 26: 579-584. Link: https://tinyurl.com/y3ssfkud
- Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, et al.( 2001) Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 34: 809-816. Link: https://tinyurl.com/yxedr883
- Réduction des risques chez les usagers de drogues (2010) Rapport. Paris: Les éditions Inserm. 573. Link: https://tinyurl.com/hy8qqz3
- Marcellin P, Péquignot F, Delarocque-Astagneau E, Zarsky JP, Ganne N, et al. (2008) Mortality related to chronic chronic hepatitis B and chronic hepatitis C in France : evidence for the role of HIV coinfection and alcohol consumption. J Hepatol 48: 200-207. Link: https://tinyurl.com/y44f65v8
- Piasecki BA, Lewis JD, Reddy KR, Bellamy SL, Porter SB, et al. (2004) Influence of alcohol use, race and viral coinfections on spontaneous HCV clearance in a US veteran population. Hepatology 40: 892-899. Link: https://tinyurl.com/yyjywusq
- Roudot-Thoraval F, Bastie A, Pawlotsky JM, Dhumeaux D (1997) Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6,664 patients. The Study Group for the Prevalence and the Epidemiology of Hepatitis C Virus. Hepatology 26: 485-490. Link: https://tinyurl.com/y3fdyvgq
- Foucher J, Reiller B, Julien V, Leal F, Scotto di Cesare E, et al. (2009) FibroScan used in street based outreach for drug users is useful for hepatitis C screening and management: a prospective study. J Viral Hepat 16: 121-131. Link: https://tinyurl.com/y2u4yez4
- de la santé (2008) Circulaire DGS/MC2 n° 2008-79 du 28 février 2008 relative à la mise en place des centres de soins, d’accompagnement et de prévention en addictologie et à la mise en place des schémas régionaux médico-sociaux d’addictologie. 15: 186-207. Link: https://tinyurl.com/y3fd987z
- Delile JM, Reiller B, Foucher J, de Ledinghen V, Gachie JP (2008 ) Hépatite C chez les usagers de drogue. Comment améliorer l’efficacité de la préventionet de la prise en charge? Alcoologie et Addictologie 30: 385-394. Link: https://tinyurl.com/yye7qybd
- de Ledinghein V, Vergniol J (2008) Transient elastography (FibroScan). Gastroenterol Clin Biol 32: 58-67. Link: https://tinyurl.com/yys2fznz
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, et al. (2010) Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51: 454-462. Link: https://tinyurl.com/y5ekpmmt
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders DSM-5 (5th Edition). Link: https://tinyurl.com/ya668tbh
- Townsend P (1987) Deprivation. Journal of Social Policy 16: 125-146. Link: https://tinyurl.com/y57wk4t8
- Meffre C, Le Strat Y, Delarocque-Astagneau E, AntonaD, Desenclos JC, et al. (2006) Institut de veille sanitaire. Link: https://tinyurl.com/y5ud7mp3
- Dhumeaux D (2014) Prise en charge des personnes infectées par les virus de l’hépatite B ou de l’hépatite C. Rapport de recommandations 2014, sous la direction du Pr Daniel Dhumeaux et sous l’égide de l’ANRS et de l’AFEF. Paris: EDP Sciences. 537. Link: https://tinyurl.com/yxhmdsm3
- Nouvelle circulaire sur la prise en charge des AAD du 10 Août 2016. INSTRUCTION N° DGOS/PF2/DGS/SP2/PP2/DSS/1C/2016/246 du 28 juillet 2016 relative à l’organisation de la prise en charge de l’hépatite C par les nouveaux antiviraux d’action directe (NAAD).
- Link: https://tinyurl.com/y2hv37ev
- Kattakuzhy S, Wilson E, Sidharthan S, Sims Z, McLaughlin M, et al. (2016) Moderate Sustained Virologic Response Rates With 6-Week Combination Directly Acting Anti-Hepatitis C Virus Therapy in Patients With Advanced Liver Disease. Clin Infect Dis 62: 440-447. Link: https://tinyurl.com/y5q94wzb
- Chevaliez S, Pawlotsky JM (2011) Méthodes alternatives au prélèvement sanguin pour le diagnostic de l’infection par le virus de l’hépatite C. Link: https://tinyurl.com/yxb2z63y
- Chevaliez S (2013) New markers for diagnosis and management of chronic hepatitis C virus infection. Ann Gastroenterol 26: 98-99. Link: https://tinyurl.com/y54s8y7k
- Cadet-Taïrou A, Gandilhon M, Martinez M, Néfau T (2015) Substances psychoactives en France: tendances récentes (2014-2015). Link: https://tinyurl.com/yyahvrjx
- Pioche C, Pelat C, Larsen C, Desenclos JC, Jauffret-Roustide M, et al. (2016) Estimation de la prévalence de l’hépatite C en population générale, France métropolitaine 2011. Bull Epidémiol Hebd 13,14: 224-229. Link: https://tinyurl.com/yysfukhq
- Vaux S, Pioche C, Brouard C, Pillonel J, Bousquet V, et al. (2017) Surveillance des hépatites B et C. Santé publique France 28. Link: https://tinyurl.com/y5spg6oy
- Larsen C, Bousquet V, Delarocque-Astagneau E, Pioche C, Roudot-Thoraval F, et al. (2010) Hepatitis C virus genotype 3 and the risk of severe liver disease in a large population of drug users in France. J Med Virol 82: 1647-1654. Link: https://tinyurl.com/yxertcym
- Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, et al. (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128: 343-350. Link: https://tinyurl.com/y4a4lshl
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, et al. (2006) Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 55: 403-408. Link: https://tinyurl.com/y5non9ps
- Boursier J, de Ledinghen V, Leroy V, Anty R, Francque S, et al. (2017) A stepwise algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J Hepatol 66: 1158-1165. Link: https://tinyurl.com/y25eo8e6

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
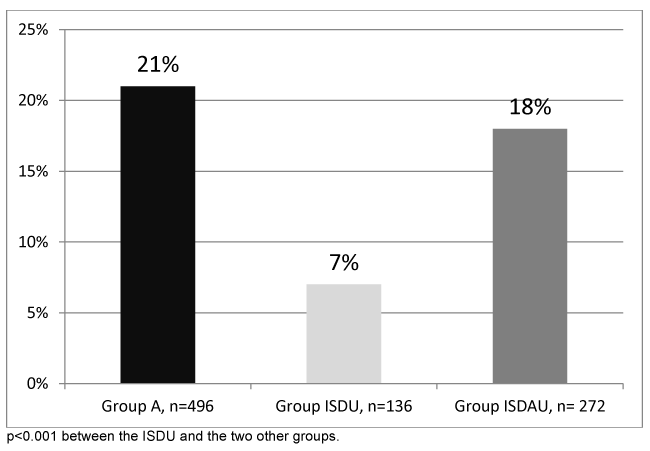
 Save to Mendeley
Save to Mendeley
