Open Journal of Hepatology
Do we really need nutraceutical liver support? Experimental effectiveness, risks and proven clinical benefits
Francesco Marotta1*, Aldo Lorenzetti1, Saida Rasulova1, Baskar Balakrishnan2, Anna Cabeca3 and Fang HE4
2Department of Immunology, Mayo Clinic, Rochester, MN, USA
3Preventive and Functional Medicine Center Brunswick, GA, USA
4Department of Nutrition and Food Hygiene, West China School of Public Health, Sichuan University, Chengdu, Sichuan, China
Cite this as
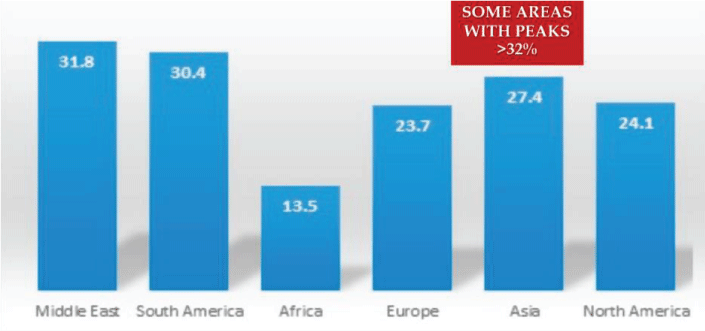
Marotta F, Lorenzetti A, Rasulova S, Balakrishnan B, Cabeca A, et al. (2021) Do we really need nutraceutical liver support? Experimental effectiveness, risks and proven clinical benefits. Open J Hepatol 3(1): 001-004. DOI: 10.17352/ojh.000006Liver is the largest and one of the most metabolically active organ in the body. With a crucial role in the clearance of toxins such as aflatoxins, microbes and metabolic by-products. This constant exposure to inner and environmental harmful substances may be potentially overwhelmed and be affected by a degree of liver damage ranging from hepatitis and Non-Alcoholic Fatty Liver Disease (NAFLD), Non-Alcohol Steatohepatitis (NASH), These latter two with worrying worldwide expansion (Figure 1) up to liver cirrhosis and hepatocellular carcinoma while representing a risk factor for other illnesss such as cardiovascular disease, stroke and diabetes.
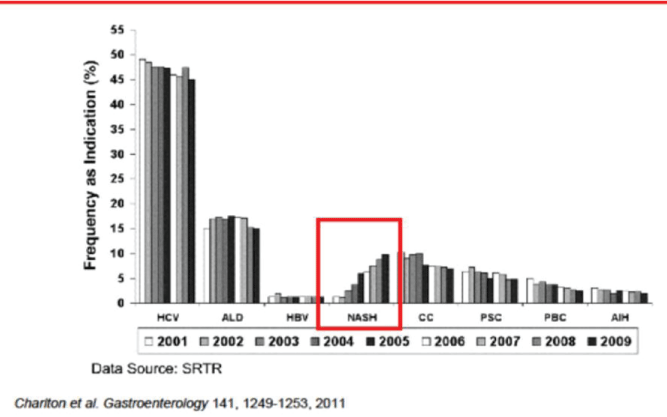
As a matter of fact, the progression from NAFLD to NASH complicated by metabolic syndrome are now representing the emergening and most rising cluster of liver disease leading to liver transplant (Figure 2).
As for liver support, there is a huge mass of empyrical data coming since the old roots of Traditional Chinese Medicine (TCM) [1], using a multitude of herbal ingredients, variably combined and prepared or associated with other Chinese medicine methods (acupuncture, moxibustion etc.). and sequential protocols. While these old roots surely contained pearls of wisdom, in modern medicine can hardly be assessed and, under no circumstance, cannot be certainly nor replicated neither advised bona fide. This is also because the major main limitation of when trying to transfer old data from TCM to a modern understandable and classifiable setting resides in the lack of knowledge of their multifaceted mechanisms of action and the detailed composition of all extracts modieties. The namber of foodstuff, funtional foods, teas, vegetable oils, and herbal extracts powered by marketing OTC claims to be supporting liver health but unapproved by health authorities is countless, although some of these with published data [2-5]. At the same time, these OTCs are hardly endowed by clear warning as for dosages and interactions with other supplement or drugs. Thus, stores dysplaying luring bottles boasting liver health support may in some unlucky circumstances act as a poison (Figure 3).
Several herbal compounds have been well studied on experimental level in vitro or in vivo but then from such studies no clinical confirmation has even been produced [6-8], or failed to confirm any significant benefit when tested clinically as in the case caffeine, Tetrandrine, resveratrol, andrographolide, curcumin and Danshen extracted from Salvia miltiorrhiza radix [9-11] (Figure 4).
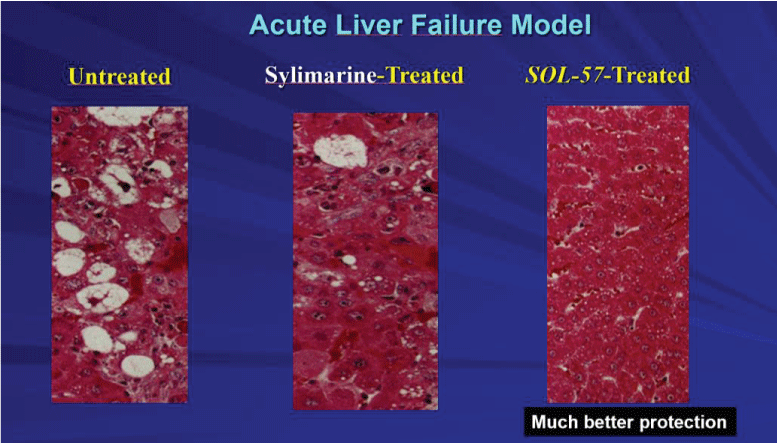
Of course, one cannot rule out that some of the prior disappointing clinical studies suffered from methdological design of by not properly organised multiple assessment as in the case of meta-analysis. In other case, highly commercialised alleged “liver protector” such as shisandra, has been reported to potential bring about side effect on lipid profile [12]. This would be a paradoxical even considering that the most common liver disease is fatty liver which may have a progressive nature [13] and associated to other metabolic syndrome dysfuntions [14-17]. Also Gamboge has blurred its popularity after serious concerns were raised for its potential damage on the redox and inflammatory citokines balance. Also some ginsenosides, extremely popular as a thole,for a range of health benefit may need a careful attention. For eample, some ginsenosides may trigger drug hepatoxicity by inhibiting CYP450 [18]. Two important Japanese compounds such as glycyrrhyzin and sho-saiko-to have almost been abandoned, the first for its unsuitable mandatory intravenous administration and the latter for some severe side effects which have caused its withdrawal from western countries [18]. A minor Japanese compound (but, in reality, a China-imported mixture of panax-ginseng, Eucommia Ulmoides, polygonati rhizome and glycyrrhiza not properly batch-to-bath controlled nor precisely specified in the label), YHK after 10 years of basic science studies [20-23] has disappeared from institutional hospitals and clinics, being unable to produce and publish any sound clinical study and, as a mater of fact, was recently shown on experimental level to be less efficient than an antioxidant complex [24]. Berberine and sylimarin are somewhat good candidates, both being very safe. The former has still a limited but promising clinical studies support [25], while the latter is the most used in the western countries, although of TCM origin. The problem of silymarin is that, although is FDA approved to treat hepatotoxicity, out of a lot of conflicting studies as for its robust significant effect, due to an erratic pharmacokinetis and unpredictable absorption [24], so far meta-analysis have assigned this drug an unclear indication [26]. A most recent promising hepatoprotector was found at an experimental level to be significantly better than sylimarine (Figure 5).
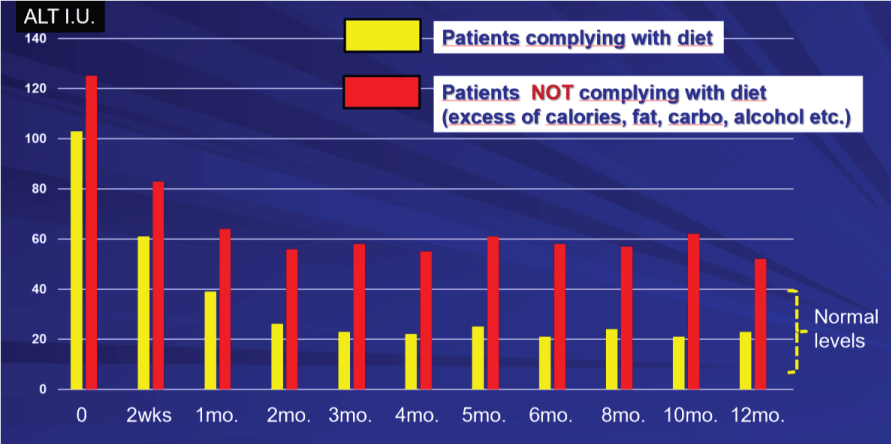
We further tested it in a variety of liver diseases, such as alcohol-related hepatitis, viral hepatitis, drug-induced hepatitis and, particularly, in NAFLD, NASH patients. This is novel mixture obtained by a detailed high-technology powered extraction and purification and isolating the most functionally active moieties (SBF: Specific Bioactive Fractions). This phytomixture containing SBF-isolated effective components (oleanolic acid and saponins-rich ginsenosides, lignans, phenolics and steroids-rich eucommiaceae extracts and alpha-OH-ursolic triterpene derivative) linked to a glyco-carrier was tested in a large multicenter study with significant benefits in liver profile and in epigenetic modulation of key genes for liver functions [27] (Figure 6).
While pharmacological treatment of viral hepatitis have made relevant steps ahead, the scenario of non-viral, benign liver dysfuntion is rapidly increasing in the last decades. This implies, besides better diet- and life-style approach, a wider involvement of “protecting” natural liver supplements. However, in the quest for giving ground to the clinical application to natural compound, pharma-grade of natural plants selecton, extraction and batch-to-batch control are mandatory. Moreover, newer molecular biology techhnologies nowadays available to improve and deepen the scrutiny of the mechanisms of action and interaction of natural compounds. While old traditional medicine reports maintain a source of hints for the medical field, they need to be taken as an impulse to advance and fine tune more modern and safe therapeutic weapons. The use of natural compounds, either used as alone or in adjunt to pharmacological treatments do represent a fascinating scenario in human health.
Conflict of interest
The authors declare that the research and the present article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The clinical study was fully supported by ReGenera R&D International for aging International (www.regenerainternational.com) which is a EU-registered not for profit research organization and by donations from Liver Support patients associations.
- Fields A (1951) Yin and Yang in ancient Chinese medicine. West J Surg Obstet Gynecol 59: VIII. Link: http://bit.ly/3eyYXoT
- Pathak S, Catanzaro R, Vasan D, Marotta F, Chabria Y, et al. (2020) Benefits of aged garlic extract in modulating toxicity biomarkers against p-dimethylaminoazobenzene and phenobarbital induced liver damage in Rattus norvegicus. Drug Chem Toxicol 43: 454-467. Link: http://bit.ly/3ru34G5
- Catanzaro R, Zerbinati N, Solimene U, Marcellino M, Mohania D, et al. (2016) Beneficial effect of refined red palm oil on lipid peroxidation and monocyte tissue factor in HCV-related liver disease: a randomized controlled study. Hepatobiliary Pancreat Dis Int 15: 165-172. Link: http://bit.ly/2MXss8a
- Marotta F, Yoshida C, Barreto R, Naito Y, Packer L (2007) Oxidative-inflammatory damage in cirrhosis: effect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol 22: 697-703. Link: http://bit.ly/3br0Ulc
- Kantah MK, Kobayashi R, Sollano J, Naito Y, Solimene U, et al. (2011) Hepatoprotective activity of a phytotherapeutic formula on thioacetamide--induced liver fibrosis model. Acta Biomed 82: 82-89. Link: http://bit.ly/3rwwAei
- Liu Z, Peng Q, Li Y, Gao Y (2018) Resveratrol enhances cisplatin-induced apoptosis in human hepatoma cells via glutamine metabolism inhibition. BMB Rep 51: 474-479. Link: http://bit.ly/3t5Egoa
- Lee KW, Shin D (2018) A Healthy Beverage Consumption Pattern Is Inversely Associated with the Risk of Obesity and Metabolic Abnormalities in Korean Adults. J Med Food 21: 935-945. Link: http://bit.ly/30DwGFv
- Elmansi AM, El-Karef AA, Shishtawy MMEl, Eissa LA (2017) Hepatoprotective Effect of Curcumin on Hepatocellular Carcinoma Through Autophagic and Apoptic Pathways. Ann Hepatol 16: 607-618. Link: http://bit.ly/3t1RORH
- Wang X, Chen Y, Han QB, Chan CY, Wang H, et al. (2009) Proteomic identification of molecular targets of gambogic acid: role of stathmin in hepatocellular carcinoma. Proteomics 9: 242-253. Link: http://bit.ly/3rvuewt
- Zhang Z, Liu T, Yu M, Li K, Li W (2018) The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J Exp Clin Cancer Res 37: 7. Link: http://bit.ly/2PHT2mN
- Cheng CY, Su CC (2010) Tanshinone IIA inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and GADD153 protein expression. Int J Mol Med 26: 379-385. Link: http://bit.ly/30pF9vH
- Le ong PK, Ko KM (2016) Schisandrin B: A Double-Edged Sword in Non-alcoholic Fatty Liver Disease. Oxid Med Cell Longev 2016: 6171658. Link: http://bit.ly/3rwxbwy
- Catanzaro R, Sciuto M, Lanzafame C, Balakrishnan B, Marotta F (2020) Platelet to lymphocyte ratio as a predictive biomarker of liver fibrosis (on elastography) in patients with hepatitis C virus (HCV)-related liver disease. Indian J Gastroenterol 39: 253-260. Link: http://bit.ly/2O7RFO2
- Kim TW (2016) Ginseng for Liver Injury: Friend or Foe? Medicines (Basel) 3: 33. Link: http://bit.ly/2PPOE5v
- Catanzaro R, Sciuto M, He F, Singh B, Marotta F (2020) Non-alcoholic fatty liver disease: correlation with hyperuricemia in a European Mediterranean population. Acta Clin Belg 19: 1-6. Link: http://bit.ly/38n0SZL
- Catanzaro R, Cuffari B, Italia A, Marotta F (2016) Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol 22: 7660-7675. Link: http://bit.ly/38pKkA2
- Catanzaro R, Calabrese F, Occhipinti S, Anzalone MG, Italia A, et al. (2014) Nonalcoholic fatty liver disease increases risk for gastroesophageal reflux symptoms. Dig Dis Sci 59: 1939-1945. Link: http://bit.ly/2OBppTy
- Tomioka H, Hashimoto K, Ohnishi H, Fujiyama R, Sakurai T, et al. (1999) An autopsy case of interstitial pneumonia probably induced by Sho-saiko-to. Nihon Kokyuki Gakkai Zasshi 37: 1013-1018. Link: http://bit.ly/30r3p0w
- Marotta F, Yadav H, Gumaste U, Helmy A, Jain S, et al. (2009) Protective effect of a phytocompound on oxidative stress and DNA fragmentation against paracetamol-induced livr damage. Ann Hepatol 8: 50-56. Link: http://bit.ly/2PHTAZT
- Catanzaro R, Celep G, Illuzzi N, Milazzo M, Rastmanesh R, et al. (2014) Anti-inflammatory and anti-mutagenic effect of the YHK phytocompound in hepatocytes: in view of an age-management liver-protecting approach. Rejuvenation Res 17: 168-171. Link: http://bit.ly/38lT7mP
- Marotta F, Lecroix P, Harada M, Masulair K, Safran P, et al. (2006) Liver exposure to xenobiotics: the aging factor and potentials for functional foods. Rejuvenation Res 9: 338-341. Link: http://bit.ly/3v5CgOP
- Marotta F, Harada M, Goh K, Lorenzetti A, Gelosa F, et al. (2006) Phytotherapeutic compound YHK exerts an inhibitory effect on early stage of experimentally-induced neoplastic liver lesions. Ann Hepatol 5: 268-272. Link: http://bit.ly/3l5Thnu
- Kantah MK, Kumari A, He F, Sollano J, Alagozlu H, et al. (2016) An Orally-Bioavailable Glutathione-Based Hepatoprotective Compound in Experimental Acute Liver Injury: More Effective than Silymarin and YHK. J Gastrointest Dig Syst 6: 462. Link: https://bit.ly/3bpKlpD
- Feng WW, Kuang SY, Tu C, Ma ZJ, Pang JY, et al. (2018) Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. Biomed Pharmacother 99: 325-333. Link: http://bit.ly/3eyZWp5
- Li WY, Yu G, Hogan RM, Mohandas R, Frye RF, et al. (2018) Relative Bioavailability of Silybin A and Silybin B From 2 Multiconstituent Dietary Supplement Formulations Containing Milk Thistle Extract: A Single-dose Study. Clin Ther 40: 103-113. Link: http://bit.ly/3buNrsu
- Hadi A, Pourmasoumi M, Mohammadi H, Symonds M, Miraghajani M (2018) The effects of silymarin supplementation on metabolic status and oxidative stress in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials. Complement Ther Med 41: 311-319. Link: http://bit.ly/3elq2LT
- Pathak S, Sruthi KH, Priya T Sathya1 T, Sato Y, et al. (2019) Environmental and dietary metabolic stress in workers: Novel avenues in oral heavy metal chelation and fatty liver aids. Nova Publisher, New York.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
