Journal of Food Science and Nutrition Therapy
Virological Water Quality
Sahar Abd Al-Daim*
Environmental Virology Lab 176, Water Pollution Research Department, Environment and Climate Change Institute, National Research Centre, 12622-Dokki, Cairo, Egypt
Cite this as
Al-Daim SA. Virological Water Quality. Food Sci Nutr The. 2025;11(1):001-013. Available from: 10.17352/jfsnt.000054Copyright License
© 2025 Al-Daim SA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Water is one of the most important environmental elements for all living creatures on earth. Attention to water quality is essential to maintaining ecosystems and life; consideration for this issue has increased on a global scale. Wastewater has a significant impact on public health because it reflects society’s progress. Wastewater-Based Epidemiology (WBE) has become a popular surveillance technique, especially in regions that are more vulnerable, for early outbreak detection, trend tracking of infectious diseases, and real-time insights. WBE provides a thorough insight into community health and lifestyle behaviors by assisting in the tracking of pathogens, including viruses, in sewage and recreational water. Monitoring viral infections at the community level requires the use of WBE, which acts as supplemental surveillance. Nonetheless, there exists an unexplored possibility for WBE to broaden its function in monitoring public health. In order to understand the connection between viral surveillance in wastewater and public health, this study highlights the significance of drinking water safety regulations and WBE, emphasizing the necessity for its further integration into public health management, and summarizes the waterborne viruses that cause health risks to public health, and their pathogenicity. We can conclude that we must search for viral indicators and we must include a virological method in guidelines of water quality in order to protect public health.
Abbreviations
AIVs: Avian Influenza viruses; BKV: BK virus; CDC: Centre for Disease Control; DBPs: Disinfection by-products; DW: Drinking water; EU: European Union; FBVE: Foodborne Viruses in Europe; GC: Genome copies; GRRP: Groundwater Replenishment Reuse Project; HAdVs: Human adenoviruses; HAV: Hepatitis A Virus; HEV: Hepatitis E Virus; HPBV: Human Picobirnavirus; Hpvs: Human Papillomaviruses; Hpyv: Human Polyomaviruses; JCV: Polyomaviruses JC Virus; MCV: Merkel cell virus; MPXV: Monkeypox virus; NIAID: National Institute of Allergy and Infectious Diseases; NORS: National Outbreak Reporting System; NoV: Noroviruses; NTU: Nephelometric Turbidity Unit; PHEIC: Public Health Emergency of International Concern; PVs: Polioviruses; QMRA: Quantitative Microbial Risk Assessment; RW: Recreational Water; SARS: Severe Acute Respiratory Syndrome; SDG: Sustainable Development Goal; TCEQ: Texas Commission on Environmental Quality; TLR: Toll-like receptor; RV: Rotavirus; USEPA: United States Environmental Protection Agency; W.H.O.: World Health Organization; WBDOSS: Waterborne Disease and Outbreak Surveillance System; WPV: Native Wild Polioviruses.
Introduction
Water is the most important element for all human life around the earth. Water quality can significantly affect health by influencing related disease rates as well as causing outbreaks of waterborne illnesses. In order to protect public health, nations create water quality guidelines. The World Health Organization (WHO) has recognized this and created a set of normative “guidelines” that provide an authoritative evaluation of the health risks connected to water-borne exposure to health hazards and the efficacy of methods for controlling them [1]. Water, despite being a recyclable resource, requires effective management to ensure a secure supply to prevent the spread of pathogenic organisms and contaminants [2]. While advancements in water treatment processes have led to a substantial decrease in the occurrence of waterborne diseases [3], water supply and quality are still exposed by the release of untreated or inadequately treated wastewater into bodies of water, which further raises the risk of pathogen transmission [4,5]. Even though some viruses are detrimental to public health, they don’t get as much attention [6]. The primary pathway for watery enteric virus transmission is the fecal-oral route [7]. This can occur from person to person or as a result of drinking tainted water, which is dangerous for your health [8]. Viral contamination of wastewater sources can occur when influents derived from the urine, vomit, and feces of infected humans and animals are released [9]. Wastewater contains a variety of viruses, including rotaviruses (RVs), adenoviruses (AdVs), enteroviruses (EVs), polioviruses (PVs), reoviruses, noroviruses (NoVs), coronaviruses (including SARS-CoV-2), hepatitis A viruses (HAV), hepatitis E viruses (HEV), and others [6]. Water pollution is a major health risk worldwide. Since 1 in 4 people will not have access to safe drinking water by 2030, potable water is absolutely necessary everywhere on Earth [10]. Three out of ten people worldwide were unable to wash their hands in their homes with soap and water during the start of the COVID-19 pandemic. Regretfully, estimates indicate that 1.6 billion people, or about 19% of the global population, will lack access to clean water by 2030 [10]. Because wastewater statistics are not influenced by human behavior or the capabilities of the health system, they provide a less biased and more cost-effective way to measure the prevalence of viruses for the purpose of monitoring human diseases [11]. Wastewater monitoring has historically been used to identify viruses transmitted by water and fecal-oral routes [12]. Wastewater monitoring has gained momentum globally due to innovative research and developments in respiratory pathogen surveillance during the COVID-19 pandemic [12,13,14]. The objective of the current review is to summarize the waterborne viruses that cause health risks to public health, and their pathogenicity, suggest strategies for virus awareness, guidelines for safe water resources, and responses owing to the consumption of polluted water.
Water quality
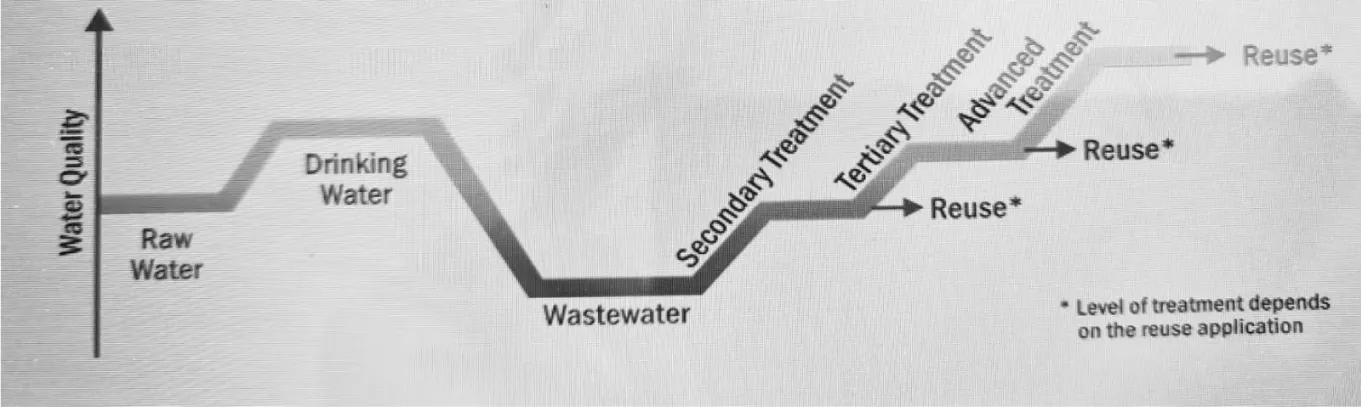
Sewer networks (SNs), sometimes referred to as wastewater networks, are an essential part of urban infrastructure since they act as channels for stormwater and wastewater to be processed or disposed of at Wastewater Treatment Plants (WWTPs). These networks are essential for maintaining the urban water environment, protecting public health and safety, and lowering the risk of waterborne illnesses as well as preventing urban flooding [15]. However, a number of problems are increasingly calling into question the effectiveness of SNs, including population growth, changes in the climate, shifts in the patterns of pollution discharge, and changing human activity [16]. The epidemics of infectious diseases associated with extreme weather events, which are frequently made worse by weakened drinking water supplies as in Figure 1, deficits in water supply and sanitation, highlight the serious effects of these issues on water quality [17,18]. Inadequate water, sanitation, and hygiene (WaSH) practices are linked to significant mortality from diarrheal infections, according to estimates from the World Health Organization (WHO), especially in low- and middle-income nations [19,20]. It is critical to comprehend the complex interactions that exist between climate change and water quality. The quantity and quality of water resources can be significantly impacted by long-term changes in temperature, precipitation patterns, and sea levels [18]. In order to address these issues, wastewater management and treatment must be prioritized. In order to improve ambient water quality, Sustainable Development Goal (SDG) goal 6.3 highlights the necessity of lowering the percentage of untreated wastewater discharged into the environment by 2030 [21]. Given the substantial impact of poorly treated wastewater on environmental deterioration, efficient wastewater management techniques are essential to reducing water pollution [22].
Impact of water quality on human health
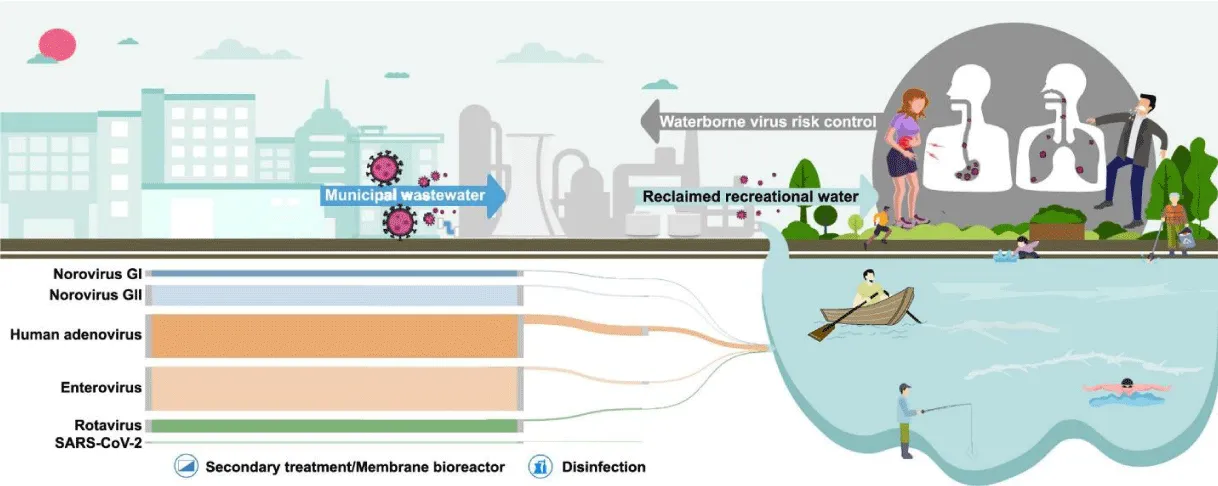
Drinking water can provide a significant health risk, with infectious disorders caused by pathogenic viruses being found in it as in Figure 2. There is a wide range of infections that can spread via polluted drinking water. Drinking water is just one of the vehicles via which infections spread via the fecal-oral pathway. Food, hands, utensils, and clothes contamination can also be an issue, especially in homes with inadequate sanitation and hygiene [23].
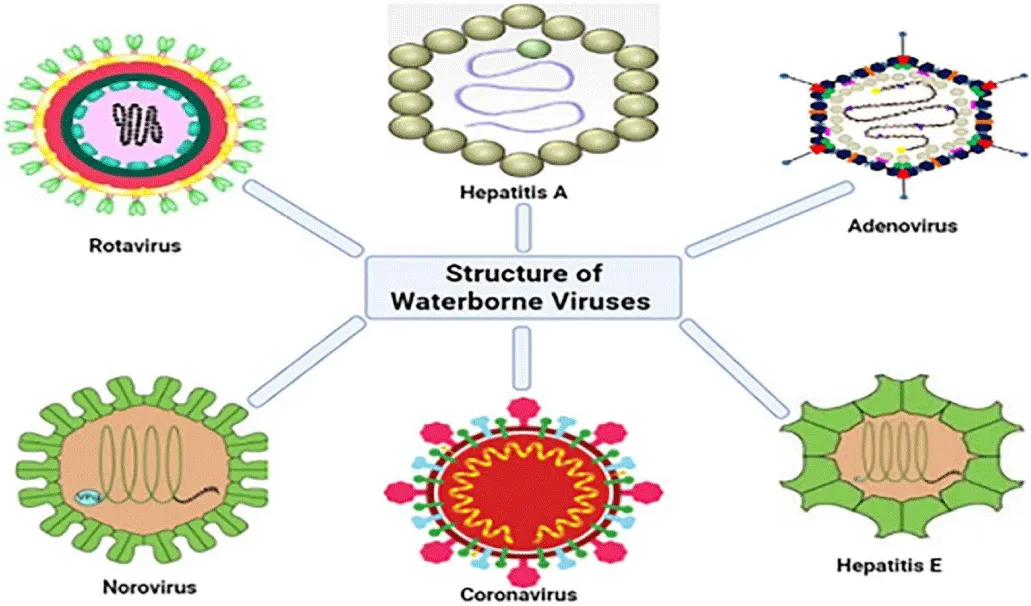
More than 150 enteric viruses are linked to water diseases globally. Hepatitis A Virus (HAV), Adenovirus (AdV), Rotaviruses (RV) and Enteroviruses (EVs), Astroviruses (AstVs), and Noroviruses (NoVs), as shown in Figure 3, are frequently found in surface waters, dams and treated drinking water [24], treated wastewater from wastewater treatment plants [25], and post-chlorinated water is not exempted [26].
A NoV infection causes nausea, cramping in the abdomen, vomiting, diarrhea, and a low fever. While the condition usually has a short duration and is self-limiting, recent data indicates that it can sometimes be fatal and severe, especially in susceptible groups such as small children, the elderly, and those with impaired immune systems. Enterovirus infections can have a wide range of health impacts, between moderate and potentially fatal. Acute gastroenteritis, which is less prevalent, fever, lethargy, sore throat, vomiting, rash, and upper respiratory tract infections are examples of mild symptoms [16]. It is possible for viraemia to develop, which can spread enteroviruses to different target organs and lead to potentially dangerous side effects such as myocarditis, encephalitis, meningitis, and poliomyelitis. A HAV infection can induce liver damage in addition to a host of other symptoms like fever, malaise, anorexia, nausea, and stomach pain that is followed by jaundice. HEV’s highly varied clinical symptoms include gastroenteritis, pneumonia, urinary tract infections, conjunctivitis, hepatitis, myocarditis, and encephalitis, as mentioned in Table 1 in addition to upper and lower respiratory tract infections. Particularly in old, immunocompromised, and pediatric individuals, adenoviruses can cause serious or even fatal illnesses. Among the clinical indications and symptoms of a human AIV virus infection are diarrhea, vomiting, fever, and stomach pain. According to research conducted by [7,16,27-29].
The WHO estimated in 2003 that infections spread through waterways resulted in 3.4 million deaths worldwide, while the EU estimates that infections spread through waterways cause 13,548 deaths annually in children under the age of 14; however, it is challenging to determine the true impact of viruses among all pathogens [29]. Diarrhea is a major cause of morbidity and death worldwide, with an estimated 4 billion instances of disease and 1.8 million fatalities per year from waterborne diarrhea [30]. Almost 90% of diarrhea-related deaths worldwide were caused by poor sanitation, inadequate hygiene, and most importantly drinking contaminated water. In addition to killing individuals, a lack of clean water damages the world economy by more than 260 billion dollars annually [31]. Outbreaks of waterborne sickness can happen even in countries with well-established waste and water treatment systems. Throughout 2009–2010, for instance, the United States of America documented at least 33 outbreaks related to DW [32]. Regardless of a country’s socioeconomic status, illnesses caused by contaminated drinking water are considered to be significantly underreported because people do not seek medical attention for infections that go away on their own and because there are currently limitations on the clinical detection of virus infections [33]. According to Albinana-Gimenez, et al. [34], the range of virus Genome Copies (GC) L−1 in raw domestic wastewater is roughly 105 to 107. The low removal efficiency of viruses caused by their sizes is one of the problems with standard water and wastewater treatment methods. This problem can be addressed by using membrane-based technologies, as noted by Ibrahim, et al. [35].
Waterborne viruses
Severe hepatitis and gastrointestinal diseases epidemics are frequently caused by viruses that spread through water. The epidemics are associated with the use of water for drinking, leisure, and recharging aquifers. The main reasons for these outbreaks, depending on the kind of water, are either insufficient or irregular drinking water treatment or the entry of feces into the water [29]. However, only 20% to 80% of enteric viruses are rendered inactive by wastewater treatment, leaving a sizable viral burden in groundwater, seawater, and river water [36]. The following list includes the viruses that are most commonly spread by water
Coronavirus 
Coronaviruses are encapsulated, single-strand RNA viruses that range in size from 60 to 220 nm. They are members of the Coronaviridae family. Fecal-oral transmission is a possible mode of infection. Concerns regarding watery transmission of coronaviruses were unfounded until the identification of a new coronavirus that produces severe acute respiratory syndrome (SARS) within sick people’s excrement. A series of closely related incidents provide more proof that sewage or ventilation systems could contaminate the surrounding area [37]. A study was recently carried out to examine the persistence of common coronaviruses in wastewater and tap water [38]. The inactivation of coronaviruses was significantly influenced by the temperature, the amount of organic matter present, and the presence of bacteria in the test water. Coronaviruses were not as fast inactivated in water at 23 °C (10 days) as they were in water at 4 °C (> 100 days); in wastewater, they were killed off in 2-4 days. Research from Germany and Brazil has shown that there is a temporal relationship, with lead durations ranging from 7 to 14 days, between the observed amounts of SARS-CoV-2 RNA in wastewater and the subsequent rise in clinical cases [39]. Similarly, in Mexico, a correlation has been shown between virus titers in wastewater and the corresponding trends in clinical cases, demonstrating the predictive power of WBE in monitoring disease dynamics. Standardizing WBE techniques for SARS-CoV-2 detection is still a major hurdle, despite its potential [40]. Standardization initiatives are critical to enhancing the consistency and dependability of wastewater-based surveillance, which is necessary to guarantee that it continues to be efficient in addressing the public health risks that viral outbreaks provide both today and, in the future, [41]. In addition, the high frequency of asymptomatic infections has highlighted the necessity of full epidemiological surveillance methods in order to efficiently track and manage the virus’s spread [42]. Reports of coronaviruses detected in wastewater or sewage in China, USA, Netherlands, Australia, France, Spain, Italy, Japan, Pakistan, India, and Turkey [43].
Monkeypox virus 
The 200–250 nm double-stranded DNA virus known as the monkeypox virus (MPXV) is encased in a lipoprotein membrane [44]. Poxviridae family, and genus Orthopoxvirus [45,46]. It was first discovered in 1970 when it was isolated from humans in a 9-year-old child from the Democratic Republic of the Congo [47]. Small mammals have been linked to the zoonotic transmission of MPXV, which is still common in West and Central Africa [48], but the 2022 global monkeypox outbreak brought with it a special circumstance. The disease initially manifested in countries where there was no prior record of cases and no epidemiological linkage to regions believed to be endemic for monkeypox. On July 23, 2022, the monkeypox virus which infects roughly 20,000 individuals worldwide was deemed a global health emergency of international importance [45,49]. Since then, this zoonotic disease has spread to more than 110 non-African nations [50]. WHO designated the global monkeypox epidemic as a Public Health Emergency of International Concern (PHEIC) on July 23, 2022 [50], 42 persons have lost their lives to the disease, and 78,000 cases have been verified in 110 locations worldwide (102 of which had no prior history of the illness) [51,52]. MPXV can enter the sewage system by discharging home water that contains respiratory secretions, saliva, and skin sores created after bathing [53]. MPXV DNA was discovered in 63 out of 312 samples that were evaluated when samples were taken the week of the first probable MPX human infection. This represents the first instance of MPXV in wastewater origin in Spain [54]. Furthermore, it was discovered that MPXV genomic DNA was present in wastewater samples from the Rome, Italy airport [55].
Polyomaviruses 
A member of the Polyomaviridae family is human polyomaviruses (HPyV). The size of polyomaviruses is 38–43 nm. Their enhanced resistance to UV light treatment and heat stability is believed to be attributed to their double-stranded DNA. Healthy people typically show no symptoms at all, but polyomavirus infections appear to be lifelong. However, kidney and brain tissue appear to be the locations of latent viral reactivation in the immunocompromised host, which may result in tumor growth. There have long been five strains of HPyV known to exist: JCV, BKV, MCV, WUV, and KIV. There are now four more strains known to exist [56]. Because they can cause a wide range of disorders, including progressive and deadly multifocal leukoencephalopathy (brain cancer), the polyomaviruses JC virus (JCV), BK virus (BKV), and Merkel Cell Virus (MCV) are particularly alarming [56]. Individuals who have immunosuppressive illnesses, or have received a lot of UV radiation are in danger. The incidence rates of MCV cases in the USA and Japan are 0.44 and 0.15 instances per 100,000 people, respectively; nevertheless, throughout the past 15 years, the number of cases has tripled [57]. Polyomaviruses are excreted in the urine and feces of infected people, both symptomatic and healthy, and one method they spread throughout the environment is by waterborne transmission. Urban sewage from several geographical zones has been found to contain JC and BK PyVs [58]. Bofill-Mass, et al. [58] examined the existence and traits of the human HPyVs that have lately been found in urban sewage and river waters. This study demonstrates the prevalence of the MCV virus in the community, which is closely linked to human cancer, and its ability to spread through urine or feces contaminating water sources. It was detected in 100% of wastewater samples as shown by [58].
Picobirnavirus 
The human picobirnavirus (HPBV), which is spherical and non-enveloped, is around 35 nm in size. They have been detected in a variety of hosts, both with and without GE, including humans. There have been reports of high incidence levels in wastewater samples, detected HPBV in 33% of final effluent samples and 100% of raw sewage samples [59]. It is unknown if animal waste is the main source of HPBV in aquatic habitats or if human waste is the sole contributing factor. However, just 25% of wastewater samples in recent research had HPBVs, raising questions about their appropriateness as markers of fecal contamination in water [60]. Whether these potential viruses meet the requirements to be regarded as a microbiological water quality indicator will require more investigation.
Papillomaviruses 
Every vertebrate has little epitheliotropic viruses called papillomaviruses. Human papillomaviruses (HPVs), classified into five genera (mu, nu, gamma, beta, and alpha), are members of the Papillomaviridae family. They have a small genome (around 50 nm - 60 nm) consisting of 7-8 kb of circular double-stranded DNA. Vaginal, oral, palmoplantar, and common warts are only a few of the many clinical signs that can result from papillomavirus infections, which are common. Strong evidence has been found to associate certain papillomaviruses with genital and cervical cancers [61]. Recent research indicates that epitheliotropic viruses may enter sewage systems as enteric viruses. A recent study found that the majority of sewage samples (81%) contained HPVs, with a wide range of genotypes belonging to both the alpha and beta genera, including potentially novel genotypes. In addition, cutaneous HPVs were detected along with three of the most important and congenital HPV types, namely the carcinogenic HPV-16 and the low-risk HPV-6 and HPV-11 genotypes [26]. Although comprehensive investigations are required to fully understand the health importance of HPVs in the environment, these findings pave the way for additional studies on HPV transmission through contaminated water.
Norovirus 
Member of the Caliciviridae family of RNA viruses, noroviruses (NoV) are single-stranded, non-enveloped viruses. They are presently grouped into five genogroups (GI, GII, GIII, GIV, and GV). Human infections are caused by the GI, GII, and GIV genotypes. It is increasingly accepted that human NoV is the main cause of epidemic GE in children and adults worldwide. It is responsible for over 90% of nonbacterial GE epidemics and nearly half of all GE cases worldwide [62]. A number of national and international surveillance systems have been established as a result of the availability of better detection techniques and increased awareness of the potential risks associated with this pathogen. These include the Norovirus Surveillance Network in Australia and New Zealand, Vironet in Canada, and Calicinet in the United States. Since 1999, the Foodborne Viruses in Europe (FBVE) network has been gathering epidemiological and laboratory data on NoV outbreaks throughout Europe. Drinking and recreational water contaminated by sewage has been the source of multiple outbreaks. GI noroviruses are believed to be more stable in water than GII strains because they are much more likely to have been spread through water than through other means. Noroviruses have been found worldwide in a variety of water habitats, including sewage systems, municipal water, rivers, groundwater, and recreational waters [63-68]. Norovirus can withstand temperatures below 0 °C and above 60 °C, as well as chlorination in quantities as high as 10 ppm. In various types of water (mineral, tap, and river), the NoV genome can survive for one to three months. In human volunteers, GI NoV preserved in groundwater remained infectious after two months, and one hundred eighty-eight days. 45% of recreational waterborne outbreaks are thought to be caused by NoV. We need to be concerned about the rise of NoV outbreak data in recent years. It’s unclear, though, if this is due to increased incidence or better reporting, surveillance, and identification [69,70].
Enterovirus 
The 7,500-nucleotide single-stranded RNA genome of human enteroviruses, which belong to the Picornaviridae family, is enclosed in an icosahedral capsid and is present in non-enveloped viral particles. They are made up of more than 100 serotypes, which are currently divided into four species, Human Enterovirus A-D, according to biological and molecular traits [71]. The fact that these viruses are widespread, shed by infected individuals in large quantities, and remain stable in the environment for a long time has led to their suggestion as a criterion for assessing viral pollution of environmental waters [72]. Furthermore, infectivity studies have revealed the presence of infective viruses in treated waters, suggesting possible threats to public health. Enteroviruses have been found in all types of water [73-76].
Hepatitis A virus 
Hepatovirus (HAV) is an icosahedral non-enveloped, single-stranded RNA virus that is a member of the Picornaviridae family. Different parts of the world have different rates of infection; non-industrialized nations with subpar sewage treatment and hygiene standards have the highest rates. In contrast, nations with successful immunization programs have seen a significant decrease in the number of documented instances of HAV infection. The virus can contaminate soil, water, and food, including shellfish that are taken from contaminated water. It is expelled in the feces of infected individuals. In tap water, the virus can live for up to 60 days; in river water, over 6 weeks; in groundwater, over 8 weeks; and in seawater, up to 30 weeks [77]. Water habitats include wastewaters [78] treated effluents [79], surface waters, and drinking waters where HAVs have been found. There have been documented cases of hepatitis A outbreaks among users of small private or public wells [80,81].
Adenovirus 
An enveloped virus of the icosahedral family Adenoviridae, genus Mastadenovirus, includes human adenoviruses (HAdVs). The 26–45 kb genome of these viruses is encased in an icosahedral protein shell and is composed of linear, double-stranded DNA. Biological traits are used to classify 51 serotypes into six species, A–F. Through the use of genetics and bioinformatics, new types are still being found and recognized. Globally, wastewater has been found to contain adenoviruses on a large scale. Out of all the enteric viruses examined in these matrices, several investigations found that AdV had the greatest quantities [82-84]. Approximately 106 virus particles per gram of fecal matter are found in the feces of infected persons. AdVs were found in 36.4% of samples, of which about 25% were infectious, according to a surveillance study conducted across Europe to ascertain the frequency of AdV and NoV in recreational waters. This finding supports the idea that AdVs are a reliable indicator of bathing water quality. Their double-stranded DNA may contribute to their enhanced stability in the environment. Additionally, it might strengthen their defenses against UV radiation inactivation. AdVs have been linked to outbreaks in drinking water. AdV is the second etiological agent for recreational outbreaks, after NoV [85,86], with 14 outbreaks of conjunctivitis or pharyngoconjunctival fever associated with pools, lakes, and ponds documented over a period of six decades.
Hepatitis E virus 
The positive-sense, non-enveloped RNA virus of the Hepevirus genus is the source of infections with the hepatitis E virus (HEV). GI and GII are exclusive to humans, whereas GIII and GIV are zoonotic viruses that can infect people and other animals in both industrialized and developing nations, including swine, poultry, deer, mongooses, and rabbits [87]. After onset, HEV infections typically go away in 1-6 weeks. With the exception of pregnant women, where the fatality rate might approach 20% - 25%, the rate is between 0.5 and 3%. HEV is listed on the NIAID List of Emerging and Re-emerging Diseases from the National Institute of Allergy and Infectious Diseases. Water tainted with human feces can easily spread HEV, which is disseminated by the fecal-oral route. Large-scale waterborne outbreaks with high rates of attack among young adults have been reported in areas with subpar sanitation.
Influenza viruses 
Seasonal epidemics are caused by influenza viruses, which are known to repeatedly spread throughout the human population globally. A phenomenon known as “antigenic drift” causes these viruses to gradually change as they spread. After the virus enters the trachea and bronchi through respiratory epithelial cells, it attaches itself and penetrates them. The host cell is destroyed as a result of viral replication [71]. It is believed that the faecal-oral transmission pathway or the consumption of contaminated water is the mode of transmission from wild waterfowl to wild waterfowl or between wild waterfowl and domestic poultry [88]. Open water bodies are contaminated by AIVs, which can transmit or reinfect domestic and wild bird populations [89,90].
Astrovirus 
The third most common cause of gastroenteritis is human astroviruses or HAstVs; they were discovered in 1975 [91]. These tiny viruses, which have diameters between 28 and 30 nm, are non-enveloped and have single-stranded RNA (+ssRNA) genomes with 6,400–7,900 nucleotides. Generation The members of the Astroviridae family are the Mastrovirus (MAstV) and Avastrovirus (AAstV) [92,93]. Infants and early children’s gastroenteritis is primarily caused by human astroviruses (HAstVs). They are present in marine waters, wastewater effluents, and surface and groundwaters intended for human use [32]. In patients with weakened immune systems, HAstVs have been connected to infections of the Central Nervous System (CNS), such as encephalitis and acute flaccid paralysis [94].
Aichivirus 
The Kobuvirus genus and Picornaviridae family comprise the Aichivirus (AiV). One of the major human gastroenteritis-causing viruses, it is spread through the faecal-oral route through contaminated food or water [95]. According to several studies [96,97], this virus is frequently seen in conjunction with other gastrointestinal infections. Africa, Asia, South America, and Europe have all recorded cases of aichivirus [98,99]. The virus is typically spread by human feces, either directly or indirectly, following the discharge of treated or untreated sewage.
Virological quality recommended for water
For drinking water to have a very low risk of spreading viral infection, human enteroviruses must basically be eliminated. Consumers who consume water from tainted sources face the risk of getting infected with a virus [6]. Two strategies to ensure that the risk of viral infection is kept to a minimum are:
- Provide drinking water from a source that has been verified to be free of fecal contamination
- Adequately treat fecally tainted water to reduce enteroviruses to a negligible level.
Research on viruses in drinking water has demonstrated that treating it can significantly lower the number of viruses present, while it might not be able to eradicate them entirely in very large amounts of water. Although risk analysis, epidemiology, and virology offer valuable information, it is currently insufficient to derive direct and quantitative virological criteria. Due to the expense, intricacy, and extended duration of virological study, as well as the inability of such criteria to identify the most pertinent viruses, their routine application is not found. For all sources, the median value of turbidity before terminal disinfection must not exceed 1 nephelometric turbidity unit (NTU) and must not exceed 5 (NTU) in a single sample. Diatomaceous earth filtration or filtration process demonstrated to be equivalent to virus reduction can also be used as shown in Table 2. The degree of virus reduction must be >90%. Disinfection is recommended to be used if monitoring has shown the presence of E. coli or thermotolerant coliform bacteria (https://megphed.gov.in/standards/qualitystd.htm).
Guidelines and regulation of water quality
Almost all of human history has featured the risk of disease when using recreational water. Egyptian mummies dating back almost 3,000 years have been found to contain evidence of schistosomiasis, a parasite disease that can only be acquired by coming into touch with contaminated water [100]. In order to protect recreational water from pollutants that are either naturally occurring or man-made, state and local governments in the United States pass restrictions. For treated water venues (such as swimming and wading pools), there is no federal regulatory agency or set of national standards for operating, disinfecting, or filtration restrictions. Because these swimming pool regulations are developed and implemented by state and local health agencies, there is a significant amount of variation observed across the country with regard to policy, compliance, and enforcement [33]. In order to verify the effectiveness of each treatment when the treatment system is put into action, the World Health Organization [101] advises guideline users to assess the reduction or inactivation of adenoviruses, reoviruses, enteroviruses, and hepatitis A virus. According to the WHO [101] guidelines, national methods and criteria should be established by each country according to its own epidemiological, social, and economic needs.
The following reasons are specifically mentioned in the United States Environmental Protection Agency’s (USEPA) [102] recommendation as to why it is not advised to set a tolerable virus content in reclaimed wastewater (viral limit):
- Viral reduction is achieved through suitable wastewater treatment.
- It takes a lot of time and effort to identify and count viruses.
- It also takes a lot of effort and time to discover infectious viruses in water.
- Documentation of waterborne viral illnesses resulting from reclaimed water is lacking, and molecular-based virus identification does not always reveal the existence of infectious viruses.
According to a WHO recommendation, the multiple-barrier approach should be utilized to control viral infectious illnesses brought on by polluted reclaimed wastewater rather than imposing a virus limit. Several state governments in the United States have established performance targets for virus eradication in wastewater reclamation, based on the USEPA criteria for water reuse. When treated wastewater is used for groundwater recharge intended for indirect potable reuse, the state of California’s Groundwater Replenishment Reuse Project (GRRP), which has the strictest and most intimate regulations regarding recycled water, requires a 12 LR of viruses and a 10 LR of both Giardia cysts and Cryptosporidium oocysts in the wastewater [103]. In cases where wastewater treatment plant effluent is used as a source, the Texas Commission on Environmental Quality (TCEQ) has established minimum (or baseline) LR and/or inactivation targets for the DPR, which are 8, 5.5, and 6 LRs of viruses, Cryptosporidium, and Giardia, respectively. However, these baseline LR targets are only intended to serve as a starting point for the TCEQ approval process and may be modified in light of data gathered from the wastewater effluent in question [104]. The Quantitative Microbial Risk Assessment (QMRA) study that was carried out to ascertain the necessary virus reduction level for reclaimed water treatment forms the basis of the Australian recommendation [105]. Rotavirus was chosen as a representative virus for this study due to its high infectivity, prevalence, and the availability of a trustworthy dose-response model. The needed reduction level in this investigation ranged from 2.1 to 6.5 log10, depending on the level of human interaction. Once more, the national guidelines of Australia embrace the multiple-barrier concept [105], as with WHO [101] and USEPA [102]. The benefit of a multiple-barrier system is that it is unlikely for multiple process units to fail at the same time, and even in the event that one unit fails, the remaining barriers should still be able to offer enough protection. Several Australian states have established standards for virus control in wastewater reclamation, based on the Australian national guidelines [105]. For instance, the state of Victoria established a standard whereby each process unit’s effectiveness is assessed based on how well it reduces viruses [106]. Moreover, Canada’s drinking water guidelines recommend eliminating enteric viruses from surface and groundwater sources to a 4-Log reduction criterion [107,108]. The reuse of wastewater is governed by laws in a few European nations, including Cyprus, France, Greece, Hungary, Italy, Portugal, and Spain. Out of these, only France has established rules about viral contamination, requiring 2 to 4 LR of F-specific bacteriophage through reclamation procedures. Due to abundant freshwater supplies and the primarily industrial use of reclaimed water, the Netherlands has adopted a novel QMRA-based viral regulation for drinking water but does not regulate viruses in reclaimed water [109,110]. A program called NEWater uses recycled water to supply about 30% of Singapore’s water supply. In order to create NEWater, secondary treated wastewater is progressively treated using microfiltration, Reverse Osmosis (RO), and ultraviolet light. Singapore does not currently have any regulations pertaining to the quality of reclaimed water; nonetheless, NEWater has been demonstrated to be enterovirus-free and to meet WHO and USEPA drinking water standards. Water is considered safe for direct potable use even though it isn’t provided as drinking water. The early warning and action components of a well-established system for keeping an eye on and handling public health risks should be included. It should make it possible to put control measures into place quickly and identify any unusual or unexpected occurrences or events early on, enabling essential verification and confirmation.
Despite its benefits, WBE faces challenges from the government [111], technological advancements [112], and data variability from a variety of sources (e.g., rainfall [113], age illness prevalence, and waste discharge. Thus, to develop the technological processes involved in WBE, more comprehension, improvement, and international cooperation are necessary [114].
Variations of drinking water standards:
There are no universally recognized and accepted international standards for drinking water. World Health Organization publishes the guidelines for setting drinking water quality standards.
Strategical responses for waterborne viruses
There are three strategies owing to water contamination by viruses as follows:
The early warning comprises the following five steps:
Step 1. Information gathering
Step 2. Recognizing signals.
Step 3. Confirmation of the event, and public health alarm.
Step 4. Initiating a public health intervention.
Step 5. Exchange of ideas.
Rules Governing Surveillance Systems are based on
- Identify the cause of the condition, the frequency with which it manifests, and its severity.
- Local physicians are in charge of managing any epidemiological information pertaining to a patient and counseling patients who have been exposed to infectious diseases on proper hygiene.
- Regular routine quality monitoring to make sure that water distribution, treatment, and sources meet the established goals and rules.
- Manage and prevent tainting water sources
- Finding and stopping epidemics can be greatly aided by effective communication [115].
Wastewater treatment to prevent disease
The process of treating wastewater entails removing, destroying, or rendering inactive the majority of pollutants and pathogens, including enteric, corona, influenza, and monkeypox viruses. Over 90% of the million annual deaths that occur in developing countries and almost half of which are in children are attributed to diseases that are brought on by wastewater. Ingesting human or animal fecal infections is the primary cause of these deaths. Therefore, there is a need for researchers to develop and analyze standards based on a more precise framework for judging the risk associated with specific viruses in wastewater. Because of this goal, Wastewater-Based Epidemiology (WBE), a method for estimating the risk of infectious diseases in people exposed to waste-related materials, is gaining popularity. The declaration made by [116-118] pandemic and endemic viral outbreaks can be identified and monitored with this technique. The reduction of waterborne infections is aided by building or household water management practices, laws, and public health surveillance, among other preventive initiatives. Public health organizations in the US, Virgin Islands, Guam, Marshall Islands, Northern Mariana Islands, Palau, Puerto Rico, and the District of Columbia conduct investigations into waterborne disease outbreaks and voluntarily report them to the CDC via the National Outbreak Reporting System (NORS) (https://www.cdc.gov/nors/about.html). To stop enteric viruses from spreading through food, water, or other channels, wastewater must also be treated to remove the viruses. However, because of their inadequate sanitation, underdeveloped nations frequently see the spread of waterborne viruses. For example, the elimination of polio worldwide has been threatened. This is because native wild polioviruses (WPV) may have spread to nations where polio is nonexistent.
Case study
One of the primary etiologic mediators of acute hepatitis globally is hepatitis A (HAV). The epidemiology of HAV is changing globally in several different geographical areas. It is estimated that about 100 million HAV infections happen worldwide each year [119]. RVs are key contributors to severe diarrhea in newborns, which causes 9% of deaths worldwide in the under-5 age group in low-income nations. Many of these places lack proper wastewater treatment facilities and sanitary infrastructure. The present investigation was carried out at two wastewater treatment plants situated in the Greater Cairo governorate, the capital city of Egypt. The treatment technology employed by El-Gabal El-Asfar WWTP and Zenin WWTP is activated sludge, and their respective flow rates were 1,700,000 cubic meters per day (m3 / day) in El-Gabal El-Asfar and 330,000 m3 /day in Zenin. Raw sewage from a large part of Cairo Governorate is received by El-Gabal El-Asfar, while a sizable portion of the El-Giza Governorate, which is thought to be a part of larger Cairo, is received by Zenin. Two predetermined WWTPs provided two liters of each type of sewage raw, processed, and 100 grams of sludge sample [119]. From August 2022 to July 2023, 72 wastewater samples were collected each month. 50%, 16.6%, 58.6%, 41.6%, 8.3%, and 41.6% of rotavirus were found in the sludge, raw, and treated (El-Gabal EL-Asfar WWTP) and zenin WWTP, respectively. In the zenin WWTP and the EL-Gabal ELAsfar WWTP, HAV was found in 58.3%, 25%, 41.6%, 41.6%, 33.3%, and 50% of the raw, treated, and sludge as shown in Table 3. This indicates that, as a result of the two WWTPS’s prior treatment processes, raw sewage, and sludge contain more viruses than treated sewage. For example, HAV was removed from 33.3% of the zenin and 8.3% of the EL-Gabal EL-Asfar, while 33.4% of the zenin and 33.3% of the RV samples were found to be removal rate. Using various barriers or a mixed form of disinfection, we advocate more restrictions on the Egyptian WWTP, as the study we conducted revealed that the LR of viruses is not tolerable according to WHO guidelines. Because the virus is still present in the aforementioned study at a high rate even after the treatment method used in the WWTPs under examination [119,122,123].
Research gaps
More study is needed to create accurate and reproducible methods for the detection of waterborne viral pathogens in order to determine the extent of contaminated water resources, the types of viruses, and the relationships between environmental factors and viral contamination. In addition, data on water quality, prevalence, exposure, and pathogen-specific features should all be utilized to improve the analysis of quantitative microbial risk assessment (QMRA). This information can help with the understanding of control methods and health risks related to water systems. The framework for safe drinking water suggests goal-setting based on health-based targets, which is an important issue that seems to need attention.
Conclusion
In Egypt, although human enteric viruses are undoubtedly a concern for the virological quality of water, we still rely on bacterial indicator systems to warn us in the event of possible contamination. The use of bacterial indicators has been disputed for decades, and we are still at a stalemate when it comes to genuinely forecasting the existence of human viruses in water. The search for a real viral signal should go on for the sake of public health protection. Every nation should establish realistic goals tailored to the particular circumstances of the area, taking into account social, cultural, environmental, and economic aspects. These goals should be established by the national authorities.
Future recommendations
We must unify the concentration, recovery, and detection techniques used for the investigation of waterborne viruses to get toward a viral indicator. Also, we recommend applying the global virological standard to drinking water treatment plants and wastewater treatment plants to save public health. In order to prevent infection and respond to outbreaks, it is imperative for public health to identify and measure the different human viruses present in environmental water. Wastewater monitoring can be used to identify potential pathogen carriers, offer detailed information, identify the source of the infections, and provide accurate early warning if it is done on a regular basis. Still, much work needs to be done to promote approval on a large scale.
The U.S.E.P.A. has introduced Method 1615 countries should invest in wastewater infrastructure and DW distribution systems, especially in large cities, as this will likely reduce a portion of the WBDOs that are reported annually, at least in high-income countries we recommend applying it in Egypt.
- World Health Organization. Guidelines for Drinking-Water Quality, 2nd ed, Addendum to Volume 1, Surveillance and Control of Community Supplies. 2nd ed. Geneva: World Health Organization; 1998. Available from: https://www.who.int/publications/i/item/WHO-EOS-98.1
- Osuolale O, Okoh A. Human enteric bacteria and viruses in five wastewater treatment plants in the eastern cape, South Africa. J Infect Public Health. 2017;10:541–547. Available from: https://doi.org/10.1016/j.jiph.2016.11.012
- Janahi EM, Mustafam S, Parkar SFD, Naser HA, Eisa ZM. Detection of enteric viruses and bacterial indicators in a sewage treatment center and shallow water bay. Int J Environ Res Public Health. 2020;17:1–13. Available from: https://doi.org/10.3390/ijerph17186483
- Naidoo S, Laniran O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int J Environ Res Public Health. 2013;11:249–270. Available from: https://doi.org/10.3390/ijerph110100249
- Yuan T, Pian Y. Hospital wastewater as hotspots for pathogenic microorganisms spread into aquatic environment: a review. Front Environ Sci. 2023;10. Available from: https://doi.org/10.3389/fenvs.2022.1091734
- Lanrewaju AA, Enitan-Folami AM, Sabiu S, Edokpayi JN, Swalaha FM. Global public health implications of human exposure to viral contaminated water. Front Microbiol. 2022;13:981896. Available from: https://doi.org/10.3389/fmicb.2022.981896
- Bouseettine R, Hassou N, Bessi H, Ennaji MM. Waterborne transmission of enteric viruses and their impact on public health. In: Emerging and reemerging viral pathogens. Elsevier; 2020. p. 907–932. Available from: https://doi.org/10.1016/B978-0-12-819400-3.00040-5
- Upfold NS, Luke GA, Knox C. Occurrence of human enteric viruses in water sources and shellfish: a focus on Africa. Food Environ Viral. 2021;13:1–31. Available from: https://doi.org/10.1007/s12560-020-09456-8
- Dias J, Pinto RN, Vieira CB, de Abreu Corrêa A. Detection and quantification of human adenovirus (HAdV), JC polyomavirus (JCPyV) and hepatitis A virus (HAV) in recreational waters of Niterói, Rio de Janeiro, Brazil. Mar Pollut Bull. 2018;133:240–245. Available from: https://doi.org/10.1016/j.marpolbul.2018.05.031
- World Health Organization, United Nations Children Emergency Fund. Progress on household drinking water, sanitation, and hygiene 2000–2020: five years into the SDGs. Geneva, Switzerland; 2021. Available from: https://www.who.int/publications/i/item/9789240030848
- Safford HR, Shapiro K, Bischel HN. Wastewater analysis can be a powerful public health tool—if it’s done sensibly. Proc Natl Acad Sci U S A. 2022;119. Available from: https://doi.org/10.1073/pnas.2119600119
- Kilaru P, Hill D, Anderson K, Collins MB, Green H, Kmush BL, et al. Wastewater surveillance for infectious disease: A systematic review. Am J Epidemiol. 2023;192(2):305–322. Available from: https://doi.org/10.1093/aje/kwac175
- Tlhagale M, Liphadzi S, Bhagwan J, Naidoo V, Jonas K, van Vuuren L, Medema G, Andrews L, Been F, Ferreira M, Saatci L. Wastewater analysis can be a powerful public health tool—if it’s done sensibly. Proc Natl Acad Sci U S A. 2022;119. Available from: http://dx.doi.org/10.2166/wh.2022.185/86887
- Boehm AB, Hughes B, Duong D, Chan-Herur V, Buchman A, Wolfe MK, White BJ. Wastewater concentrations of human influenza, metapneumovirus, parainfluenza, respiratory syncytial virus, rhinovirus, and seasonal coronavirus nucleic acids during the COVID-19 pandemic: A surveillance study. Lancet Microbe. 2023;4(5):e340–e348. Available from: https://doi.org/10.1016/s2666-5247(22)00386-x
- Owolabi TA, Mohandes SR, Zayed T. Investigating the impact of sewer overflow on the environment: a comprehensive literature review paper. J Environ Manag. 2022;301:113810. Available from: https://doi.org/10.1016/j.jenvman.2021.113810
- Ahmed T, Zounemat-Kermani M, Scholz M. Climate change, water quality and water-related challenges: a review with focus on Pakistan. Int J Environ Res Public Health. 2020;17:851. Available from: https://doi.org/10.3390/ijerph17228518
- Nichols G, Lake I, Heaviside C. Climate change and water-related infectious diseases. Atmosphere. 2018;9:385. Available from: https://doi.org/10.3390/atmos9100385
- Nijhawan A, Howard G. Associations between climate variables and water quality in low- and middle-income countries: a scoping review. Water Res. 2022;210:117996. Available from: https://doi.org/10.1016/j.watres.2021.117996
- Prüss-Üstün A, Wolf J, Bartram J, Clasen T, Cumming O, Freeman MC, et al. Burden of disease from inadequate water, sanitation, and hygiene for selected adverse health outcomes: an updated analysis with a focus on low- and middle-income countries. Water Res. 2019;222:765–777. Available from: https://doi.org/10.1016/j.ijheh.2019.05.004
- World Health Organization. Water, sanitation and hygiene: burden of disease. Available from: https://www.who.int/data/gho/data/themes/topics/water-sanitation-and-hygiene-burden-of-disease. 2024. Available from: https://www.who.int/data/gho/data/themes/topics/water-sanitation-and-hygiene-burden-of-disease
- UN Water. Progress on wastewater treatment (SDG target 6.3). Available from: https://www.sdg6data.org/en/indicator/6.3.1. 2024.
- Jones ER, Bierkens MFP, Wanders N, Sutanudjaja EH, van Beek LPH, van Vliet MTH. Current wastewater treatment targets are insufficient to protect surface water quality. Commun Earth Environ. 2022;3:221. Available from: https://www.nature.com/articles/s43247-022-00554-y
- Avendano P, Matson DO, Long S, Whitney C, Matson C, Pickering LK. Costs associated with office visits for diarrhea in infants and toddlers. Pediatr Infect Dis J. 1993;12:897–902. Available from: https://doi.org/10.1097/00006454-199311000-00001
- Opere WM. Detection of pathogenic human adenoviruses in water samples collected from Lake Victoria along Homa Bay Town, Homa Bay County: Kenya. Kenyatta University; 2019. Available from: https://doi.org/10.1007/s12560-020-09444-y
- Gonzales-Gustavson E, Rusiñol M, Medema G, Calvo M, Girones R. Quantitative risk assessment of norovirus and adenovirus for the use of reclaimed water to irrigate lettuce in Catalonia. Water Res. 2019;153:91–99. Available from: https://doi.org/10.1016/j.watres.2018.12.070
- La Rosa G, Fratini M, Accardi L, D’Oro G, Libera S, Muscillo M. Mucosal and cutaneous human papillomaviruses detected in raw sewages. PLoS One. 2012. Available from: https://doi.org/10.1371/journal.pone.0052391
- Haramoto E, Malla B, Thakali O, Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;1,737:140405. Available from: https://doi.org/10.1016/j.scitotenv.2020.140405
- Randazzo M, Sánchez G. Hepatitis A infections from food. J Appl Microbiol. 2020;129:1120–1132. Available from: https://doi.org/10.1111/jam.14727
- Gibson KE. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr Opin Virol. 2014;4:50–57. Available from: https://doi.org/10.1016/j.coviro.2013.12.005
- Manetu WM, Karanja AM. Waterborne disease risk factors and intervention practices: a review. Open Access Libr. 2021;8:1–11. Available from: https://www.scirp.org/journal/paperinformation?paperid=109142
- World Health Organization. Global Costs and Benefits of Drinking-Water Supply and Sanitation Interventions to Reach the MDG Target and Universal Coverage. Geneva, Switzerland; 2012. Available from: https://www.who.int/publications/i/item/WHO-HSE-WSH-12.01
- Magana-Arachchi D, Wanigatunge R. Ubiquitous waterborne pathogens. In: Elsevier; 2020. p. 15–42. Available from: https://doi.org/10.1016/B978-0-12-818783-8.00002-5
- Centers for Disease Control and Prevention. Surveillance data from swimming pool inspections—selected states and counties, United States, 52:513–6. MMWR. 2002. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5222a1.htm
- Albinana-Gimenez N, Clemente-Casares P, Bofill-Mas S, Hundesa A, Ribas F, Girones R. Distribution of human polyomaviruses, adenoviruses, and hepatitis E virus in the environment and in a drinking-water treatment plant. Environ Sci Technol. 2006;40:7416–7422. Available from: https://doi.org/10.1021/es060343i
- Ibrahim Y, Ouda M, Kadadou D, Banat F, Naddeo V, Alsafar H. Detection and removal of waterborne enteric viruses from wastewater: a comprehensive review. J Environ Chem Eng. 2021;9:105613. Available from: https://doi.org/10.1016/j.jece.2021.105613
- La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanita. 2010;46:266–273. Available from: https://doi.org/10.4415/ann_10_03_07
- Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Agents Int J Antimicrob. 2020;56. Available from: https://doi.org/10.1016/j.ijantimicag.2020.106054
- Sharan M, Vijay D, Yadav JP, Bedi JS, Dhaka P. Surveillance and response strategies for zoonotic diseases: a comprehensive review. Sci One Health. 2023;100050. Available from: https://doi.org/10.1016/j.soh.2023.100050
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020. Available from: https://doi.org/10.1186/s13054-020-03120-0
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2, and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228–248. Available from: https://doi.org/10.1002/path.5471
- Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-19. Nature. 2020;581:465–469. Available from: https://doi.org/10.1038/s41586-020-2196-x
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. Available from: https://doi.org/10.1002/path.1570
- Amoah ID, Kumari S, Bux F. Coronaviruses in wastewater processes: Source, fate and potential risks, review. Environ Inter Sci Dir. 2020;143:105262. Available from: https://doi.org/10.1016/j.envint.2020.105962
- Hraib M, Jouni S, Albitar MM, Alaidi S, Alshehabi Z. The outbreak of monkeypox: an overview. Ann Med Surg. 2022;79. Available from: https://doi.org/10.1016/j.amsu.2022.104069
- Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, et al. Epidemiological, clinical, and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy. Euro Surveill. 2022;22:6–18. Available from: https://doi.org/10.2807/1560-7917.es.2022.27.22.2200421
- Von Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. A POX-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19602200105
- Marennikova SS, Mal’ceva NN, Macevit GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Epidemiol. 1972. Available from: https://pubmed.ncbi.nlm.nih.gov/4340219/
- Wolfe MK, Duong D, Hughes B, Chan-Herur V, White BJ, Boehm AB, et al. Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv. 2022. Available from: https://www.medrxiv.org/content/10.1101/2022.07.25.22278043v1.full
- Ilic I, Zivanovic Macuzic I, Ilic M. Global outbreak of human monkeypox in 2022: update of epidemiology. Trav Med Infect Dis. 2022;7:264. Available from: https://doi.org/10.3390/tropicalmed7100264
- WHO. Disease outbreak news. World Health Organization. 2023. Available from: https://doi.org/10.3390/tropicalmed7100264
- WHO. Monkeypox. World Health Organization. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Mao K, Zhang K, Du W, Ali W, Feng X, Zhang H. The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Curr Opin Environ Sci Health. 2020;17:1–7. Available from: https://doi.org/10.1016/j.coesh.2020.04.006
- Tiwari A, Adhikari S, Kaya D, Islam MA, Malla B, Sherchan SP, et al. Monkeypox outbreak: wastewater and environmental surveillance perspective. Sci Total Environ. 2023;856. Available from: https://doi.org/10.1016/j.scitotenv.2022.159166
- Giron-Guzman I, Díaz-Reolid A, Truchado P, Carcereny A, Hernaez B, Bosch A, et al. Wastewater Based Epidemiology beyond SARS-CoV-2: Spanish Wastewater Reveals the Current Spread of Monkeypox Virus. Water Res. 2022;1,231:119621. Available from: https://doi.org/10.1016/j.watres.2023.119621
- Rosa L, Ferraro B, La Rosa G. Detection of monkeypox virus DNA in the wastewater of an airport in Rome, Italy: expanding environmental surveillance to emerging threats. medRxiv. 2022. Available from: https://doi.org/10.3201/eid2901.221311
- WHO. Diarrheal disease. World Health Organization. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases. Pathol Res Int. 2011;123491. Available from: https://doi.org/10.4061/2011/123491
- Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, Hundesa A, Rodriguez-Manzano J, Allard A. Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol. 2006;72(12):7894–6. Available from: https://doi.org/10.1128/AEM.00965-06
- Symonds EM, Griffin DW, Breitbart M. Eukaryotic viruses in wastewater samples from the United States. Appl Environ Microbiol. 2009;75(5):1402–9. Available from: https://doi.org/10.1128/AEM.01899-08
- Hamza IA, Jurzik L, Uberla K, Wilhelm M. Evaluation of pepper mild mottle virus, human picobirnavirus, and Torque teno virus as indicators of fecal contamination in river water. Water Res. 2011;45(3):1358–68. Available from: https://doi.org/10.1016/j.watres.2010.10.021
- IARC (International Agency for Research of Cancer). A review of human carcinogens: biological agents. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: IARC; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK304348/
- Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009;44(1):1-8. Available from: https://doi.org/10.1016/j.jcv.2008.10.009
- Jung JH, Yoo CH, Koo ES, Kim HM, Na Y, Jheong WH. Occurrence of norovirus and other enteric viruses in untreated groundwaters of Korea. Water Health. 2011;9(3):544-55. Available from: https://doi.org/10.2166/wh.2011.142
- Williamson WM, Ball A, Wolf S, Hewitt J, Lin S, Scholes P. Enteric viruses in New Zealand drinking-water sources. Water Sci Technol. 2011;63(8):1744-51. Available from: https://doi.org/10.2166/wst.2011.117
- Mans J, Netshikweta R, Magwalivha M, Van Zyl WB, Taylor MB. Diverse norovirus genotypes identified in sewage-polluted river water in South Africa. Epidemiol Infect. 2012;1-11. Available from: https://doi.org/10.1017/s0950268812000490
- Maunula L, Soderberg K, Vahtera H, Vuorilehto VP, von Bonsdorff CH, Valtari M. Presence of human noro- and adenoviruses in river and treated wastewater, a longitudinal study and method comparison. J Water Health. 2012;10(1):87-99. Available from: https://doi.org/10.2166/wh.2011.095
- Skraber S, Langlet J, Kremer JR, Mossong J, De LS, Even J. Concentration and diversity of noroviruses detected in Luxembourg wastewaters in 2008-2009. Appl Environ Microbiol. 2011;77(15):5566-8. Available from: https://doi.org/10.1128/aem.00632-11
- Kitajima M, Oka T, Haramoto E, Phanuwan C, Takeda N, Katayama K. Genetic diversity of genogroup IV noroviruses in wastewater in Japan. Lett Appl Microbiol. 2011;52(2):181-4. Available from: https://doi.org/10.1111/j.1472-765X.2010.02980.x
- Charles KJ, Shore J, Sellwood J, Laverick M, Hart A, Pedley S. Assessment of the stability of human viruses and coliphage in groundwater by PCR and infectivity methods. J Appl Microbiol. 2009;106(6):1827-37. Available from: https://doi.org/10.1111/j.1365-2672.2009.04150.x
- Seitz SR, Leon JS, Schwab KJ, Lyon GM, Dowd M, McDaniels M. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol. 2011;77(19):6884-8. Available from: https://doi.org/10.1128/AEM.05806-11
- Stanway G, Brown F, Christian P, Hovi TY, Ypia T, King AMQ. Virus taxonomy: eighth report of the international committee on taxonomy of viruses. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Family Picornaviridae. London: Elsevier/Academic Press; 2005;757-78. https://www.sciencedirect.com/book/9780122499517/virus-taxonomy
- Hot D, Legeay O, Jacques J, Gantzer C, Caudrelier Y, Guyard K. Detection of somatic phages, infectious enteroviruses, and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Res. 2003;37(19):4703-10. Available from: https://doi.org/10.1016/s0043-1354(03)00439-1
- Ehlers MM, Grabow WO, Pavlov DN. Detection of enteroviruses in untreated and treated drinking water supplies in South Africa. Water Res. 2005;39(11):2253-8. Available from: https://doi.org/10.1016/j.watres.2005.04.014
- Lee C, Lee SH, Han E, Kim SJ. Use of cell culture-PCR assay based on combination of A549 and BGMK cell lines and molecular identification as a tool to monitor infectious adenoviruses and enteroviruses in river water. Appl Environ Microbiol. 2004;70(11):6695-705. Available from: https://doi.org/10.1128/aem.70.11.6695-6705.2004
- Haramoto E, Katayama H, Oguma K, Ohgaki S. Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Appl Environ Microbiol. 2005;71(5):2403-11. Available from: https://doi.org/10.1128/aem.71.5.2403-2411.2005
- Shieh YC, Wong CI, Krantz JA, Hsu FC. Detection of naturally occurring enteroviruses in waters using direct RTPCR and integrated cell culture-RT-PCR. J Virol Methods. 2008;149(1):184-9. Available from: https://doi.org/10.1016/j.jviromet.2007.12.013
- Rodriguez-Lazaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS. Virus hazards from food, water, and other contaminated environments. FEMS Microbiol Rev. 2011;10:6976. Available from: https://doi.org/10.1111/j.1574-6976.2011.00306.x
- Villar LM, de Paula VS, Diniz-Mendes L, Guimaraes FR, Ferreira FF, Shubo TC. Molecular detection of hepatitis A virus in urban sewage in Rio de Janeiro, Brazil. Let Appl Microbiol. 2007;45(2):168-73. Available from: https://doi.org/10.1111/j.1472-765X.2007.02164.x
- Prado T, Silva DM, Guilayn WC, Rose TL, Gaspar AM, Miagostovich MP. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011;45(3):1287-97. Available from: https://doi.org/10.1016/j.watres.2010.10.012
- Moreno S, Alvarado MV, Bermudez A, Gutierrez MF. Phylogenetic analysis indicates human origin of rotavirus and hepatitis A virus strains found in the drinking water of western Colombia. Biomedica. 2009;29(2):209-17. Available from: https://pubmed.ncbi.nlm.nih.gov/20128346/
- Hernandez-Morga J, Leon-Felix J, Peraza-Garay F, Gil-Salas BG, Chaidez C. Detection and characterization of hepatitis A virus and Norovirus in estuarine water samples using ultrafiltration--RT-PCR integrated methods. J Appl Microbiol. 2009;106(5):1579-90. Available from: https://doi.org/10.1111/j.1365-2672.2008.04125.x
- Irving NLG, Smith FA. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl Environ Microbiol. 1981;41:51-9. Available from: https://doi.org/10.1128/aem.41.1.51-59.1981
- Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009;90(1):1-20. Available from: https://doi.org/10.1099/vir.0.003087-0
- Mena KD, Gerba CP. Waterborne adenovirus. Rev Environ Contam Toxicol. 2009;198:133-67. Available from: https://doi.org/10.1007/978-0-387-09647-6_4
- Artieda J, Pineiro L, Gonzalez M, Munoz M, Basterrechea M, Iturzaeta A. A swimming pool-related outbreak of pharyngoconjunctival fever in children due to adenovirus type 4, Gipuzkoa, Spain, 2008. Euro Surveill. 2009;14(8):19125. Available from: https://pubmed.ncbi.nlm.nih.gov/19250625/
- Divizia M, Gabrieli R, Donia D, Macaluso A, Bosch A, Guix S. Waterborne gastroenteritis outbreak in Albania. Water Sci Technol. 2004;50(1):57-61. Available from: https://pubmed.ncbi.nlm.nih.gov/15318487/
- Meng XJ. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet Microbiol. 2010;27:256-65. Available from: https://doi.org/10.1016/j.vetmic.2009.03.017
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152-79. Available from: https://doi.org/10.1128/mr.56.1.152-179.1992
- Keeler SP, Dalton MS, Cressler AM, Berghaus RD, Stallknecht DE. Abiotic factors affecting the persistence of avian influenza virus in surface waters of waterfowl habitats. Appl Environ Microbiol. 2014;80(9):2910–2917. Available from: https://doi.org/10.1128/aem.03790-13
- Zhang H, Li Y, Chen J, Chen Q, Chen Z. Perpetuation of H5N1 and H9N2 avian influenza viruses in natural water bodies. J Gen Virol. 2014;95(7):1430–1435. Available from: https://doi.org/10.1099/vir.0.063438-0
- Vu DL, Bosch A, Pintó RM, Guix S. Epidemiology of classic and novel human astrovirus: gastroenteritis and beyond. Viruses. 2017;9(2):33. Available from: https://doi.org/10.3390/v9020033
- Appleton H, Higgins PG. Letter: Viruses and gastroenteritis in infants. Lancet. 1975 Jun 7;1(7919):1297. Available from: https://doi.org/10.1016/s0140-6736(75)92581-7
- Donato C, Vijaykrishna D. The broad host range and genetic diversity of mammalian and avian astroviruses. Viruses. 2017;9(5):102. Available from: https://doi.org/10.3390/v9050102
- Cordey S, Brito F, Vu D-L, Turin L, Kilowoko M, Kyungu E. Astrovirus VA1 identified by next-generation sequencing in a nasopharyngeal specimen of a febrile Tanzanian child with acute respiratory disease of unknown etiology. Emerg Microb Infect. 2016;5:1-3. Available from: https://doi.org/10.1038/emi.2016.67
- Kebe O, Fernandez-Garcia MD, Fall A, Dia H, Bidalot M, Ambert-Balay K. Prevalence and genetic diversity of Aichi virus 1 from urban wastewater in Senegal. Intervirology. 2021;64:96–101. Available from: https://doi.org/10.1159/000512130
- Koopmans M, von Bonsdorff CH, Vinjé J, de Medici D, Monroe S. Foodborne viruses. FEMS Microbiol Rev. 2002;26:187–205. Available from: https://doi.org/10.1111/j.1574-6976.2002.tb00610.x
- Japhet M, Famurewa O, Adesina O, Opaleye O, Wang B, Höhne M. Viral gastroenteritis among children of 0-5 years in Nigeria: characterization of the first Nigerian Aichi virus, recombinant noroviruses and detection of a zoonotic astrovirus. J Clin Virol. 2019;111:4–11. Available from: https://doi.org/10.1016/j.jcv.2018.12.004
- Oh DY, Silva P, Hauroeder B, Diedrich S, Cardoso D, Schreier E. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch Virol. 2006;151:1199–1206. Available from: https://doi.org/10.1007/s00705-005-0706-7
- Lodder WJ, Rutjes SA, Takumi K, de Roda Husman AM. Aichi virus in sewage and surface water, the Netherlands. Emerg Infect Dis. 2013;19:1222–1230. Available from: https://doi.org/10.3201/eid1908.130312
- Chastel C. When the Egyptian mummies are speaking about the infections that have made them ill. Hist Sci Med. 2004;38:147-155. Available from: https://pubmed.ncbi.nlm.nih.gov/15338573/
- World Health Organization (WHO). WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater. Vol 1. Policy and Regulatory Aspects. Geneva: World Health Organization; 2006.
- United States Environmental Protection Agency (USEPA). Guidelines for Water Reuse. 2012. Available from: https://www.epa.gov/sites/default/files/2019-08/documents/2012-guidelines-water-reuse.pdf
- State Water Resources Control Board. Regulations Related to Recycled Water. 2015.
- Texas Water Development Board. Final Report. Direct Potable Reuse Resource Document. 2015. Available from: https://www.twdb.texas.gov/publications/reports/contracted_reports/doc/1248321508_Vol1.pdf
- Natural Resource Management Ministerial Council (NRMMC). National Water Quality Management Strategy. Australian Guidelines for Water Recycling: Managing Health and Environmental Risks Phase 1. 2006. Available from: https://www.waterquality.gov.au/sites/default/files/documents/water-recycling-guidelines-full-21.pdf
- The State of Victoria. Guide for the Completion of a Recycled Water Quality Management Plan. For Class A Water Recycling Schemes. 2008.
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Enteric Viruses. 2012. Accessed April 21, 2023. Available from: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-enteric-viruses.html
- Health Canada. Guidelines for Recreational Water Quality: Indicators of Fecal Contamination. 2021. Accessed January 15, 2023. Available from: https://www.canada.ca/en/health-canada/programs/consultation-guidelines-recreational-water-quality-fecal-contamination/document.html
- Smeets PW, Medema GJ, van Dijk JC. The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drink Sci Water Eng. 2009;2:1–14. Available from: https://dwes.copernicus.org/articles/2/1/2009/
- Paranychianakis NV, Salgot M, Snyder SA, Angelakis AN. Water reuse in EU states: necessity for uniform criteria to mitigate human and environmental risks. Crit Rev Environ Sci Technol. 2015;45(13):1409–1468. Available from: https://doi.org/10.1080/10643389.2014.955629
- Daughton CG. Wastewater surveillance for population-wide Covid-19: the present and future. Sci Total Environ. 2020;20(736):139631. Available from: https://doi.org/10.1016/j.scitotenv.2020.139631
- Gable L, Ram N, Ram JL. Legal and ethical implications of wastewater monitoring of SARS-CoV-2 for COVID-19 surveillance. J Law Biosci. 2020;7. Available from: https://doi.org/10.1093/jlb/lsaa039
- Mainardi PH, Bidoia ED. Challenges and emerging perspectives of an international SARS-CoV-2 epidemiological surveillance in wastewater. An Acad Bras Cienc. 2021;93. Available from: https://www.scielo.br/j/aabc/a/nhyD4kCCqVqd5WpbFRQJhbM/?format=pdf&lang=en
- Pillay L, Amoah ID, Kumari S, Bux F. Potential and challenges encountered in the application of wastewater-based epidemiology as an early warning system for COVID-19 infections in South Africa. ACS ES and T Water. 2022;2:2105–2113. Available from: http://dx.doi.org/10.1021/acsestwater.2c00049
- Redwan NA, Al-Fassi FA, Ali MA. Health aspects of virological water quality: An overview review. J Appl Sci Res. 2008;4(10):1205-1215. Available from: https://www.aensiweb.com/old/jasr/jasr/2008/1205-1215.pdf
- Javanian M, Barary M, Ghebrehewet S, Koppolu V, Vasigala V, Ebrahimpour S. A brief review of influenza virus infection. J Med Virol. 2021;93(8):4638–4646. Available from: https://doi.org/10.1002/jmv.26990
- Anand A, Rajchakit U, Sarojini V. Detection and removal of biological contaminants in water: the role of nanotechnology. In: Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier; 2020:69–110. Available from: http://dx.doi.org/10.1016/B978-0-12-818489-9.00004-9
- Lin N, Servetas S, Jackson S, Lippa K, Parratt K, Mattson P. Report on the DHS/NIST Workshop on Standards for an Enduring Capability in Wastewater Surveillance for Public Health (SWWS Workshop). NIST Special Publication. 2022. Available from: https://www.nist.gov/publications/report-dhsnist-workshop-standards-enduring-capability-wastewater-surveillance-public
- Al-Daim SA. One-year investigation of hepatitis A virus and rotavirus in two wastewater treatment plants in Egypt. J Biomed Res Environ Sci. 2024;5(2):106-116. Available from: https://www.jelsciences.com/abstracts/1694
- Elsamadony M, Fujii M, Miura T, Watanabe T. Possible transmission of viruses from contaminated human feces and sewage: implications for SARS-CoV-2. Sci Total Environ. 2021;755:142575. Available from: https://doi.org/10.1016/j.scitotenv.2020.142575
- Altintas Z, Gittens M, Pocock J, Tothill IE. Biosensors for waterborne viruses: detection and removal. Biochimie. 2015;115:144–154. Available from: https://doi.org/10.1016/j.biochi.2015.05.010
- Centers for Disease Control and Prevention (CDC). Morbidity and Mortality Weekly Report. 2013;62. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6218md.htm
- Monteiro GS, Staggemeier R, Klauck CR, Bernardes AM, Rodrigues MAS, Spilki FR. Degradation and inactivation of adenovirus in water by photo-electro-oxidation. Braz J Biol. 2015;75(Suppl):S37–S42. Available from: https://doi.org/10.1590/1519-6984.00813suppl
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
