Journal of Food Science and Nutrition Therapy
Investigation of Structural Features of Prunes (Prunus domestica) Insoluble Dietary Fibers
Thaisa Moro Cantu-Jungles, Marcello Iacomini and Lucimara MC Cordeiro*
Cite this as
Cantu-Jungles TM, Iacomini M, Cordeiro LMC (2017) Investigation of Structural Features of Prunes (Prunus domestica) Insoluble Dietary Fibers. J Food Sci Nutr The 3(1): 001-006. DOI: 10.17352/jfsnt.000006Structural characteristics of dietary fibers are closely related to its biological functions in the human body. Previously, soluble dietary fibers from prunes were extracted and characterized. In this work, structural analysis of insoluble dietary fibers was conducted using monosaccharide composition, methylation, molecular weight determination and 13C-NMR data. Prunes’ non-cellulosic insoluble fibers were found to contain, a pectic type I arabinogalactan, a fucogalactoxyloglucan and a heteroxylan. These findings suggest that insoluble dietary fibers can be composed by some pectic polysaccharides besides cellulose and hemicellulosic polymers. This paper brings important structural features of insoluble dietary fibers from prunes that may be of biological significance.
Introduction
Dietary fibers are carbohydrate polymers composed by ten or more monomeric units, which are not hydrolyzed by endogenous enzymes in the small intestine and can be partially or totally fermented in the large intestine of humans[1,2] . Health benefits associated with dietary fiber consumption result from its low caloric content, physical effects in the stomach and small intestine and fermentation in the colon. Dietary fibers can be classified as either water soluble and mostly fermentable (such as pectin) or insoluble, less fermentable, and nonviscous (such as cellulose, lignin, and some of the hemicelluloses) [3].
Soluble fibers are generally known to increase viscosity of the stomach and small intestine content, improving satiety, reducing post-prandial glycaemia and preventing reabsorption of bile acids, thus reducing circulating blood cholesterol levels. Moreover, due to its high fermentability, soluble fibers can positively modulate the colonic microflora preventing pathologies such as infectious diseases, allergy or asthma, colon cancer, obesity, liver disease, diabetes and inflammatory bowel disease. On the other hand, insoluble fibers are known to be poorly fermentable, but are able to increase fecal bulk and decrease transit time, increasing stool frequency [4]. However, not all soluble/insoluble fibers behave in the same way. For example, the soluble dietary fiber inulin was shown to increase stool frequency [5]. Likewise, insoluble fibers, such as resistant starch, are highly fermented by the human gut microbiota [6]. This may be because carbohydrate polymers, as dietary fibers, represent the most heterogeneous and diverse group of associated molecules found in nature. Therefore, not only water solubility, but other structural features such as monosaccharide composition, linkage types between monosaccharides, size of the polymers, branching patterns, etc., also dictates their biological activities [6,7]. Thus, the knowledge about chemical structure of food dietary fibers is important to explore how it interacts with the human body and possibly produce health benefits.
Prunes, the dried fruits of plums (Prunus domestica), possess as high as 62.7% of carbohydrates and its consumption is related to laxative effects, reductions in cardiovascular risk and sugar metabolism control that may be associated with dietary fiber constituents [8]. We have previously carried out the isolation and characterization of soluble dietary fibers found in prunes [9] and the pectic polysaccharides homogalacturonan and rhamnogalacturonans with type I arabinogalactans side chains have been described. In this work, our objective was to further analyze the chemical structure of insoluble dietary fibers through monosaccharide composition, linkage analysis, molecular weight determination and 13C-NMR data and thus expand our knowledge about their structural characteristics.
Materials and Methods
Plant material
Pitted prunes (Prunus domestica) from cultivar d’Agen were purchased at local market in Curitiba (Brazil) (LA VIOLETERA®).
Extraction and purification of polysaccharides
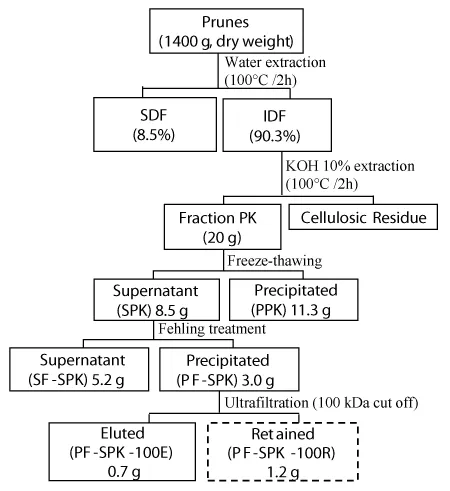
Prunes (2 kg) were blended and exhaustively extracted with water (6 L) at 100 ºC under reflux for 2 h as previously described [9] to remove soluble dietary fibers (SDF). The residue of hot water extraction, containing the insoluble dietary fibers (IDF), was separated after centrifugation (8000 rpm, 15 min at 15 °C). To solubilize some of the polysaccharides present in the IDF, mainly hemicelluloses, the residue was submitted to alkaline extraction with KOH 10% (2 L each, 3x) at 100 ºC under reflux for 2 h. Alkaline extract was then obtained by centrifugation (8000 rpm, 15 min at 15 °C), followed by neutralization with HOAc, dialysis and lyophilization, resulting in a polysaccharide fraction named herein as PK (prunes’s alkaline extract) (Figure 1). The residue remaining of this extraction contained cellulose that has not been solubilized with this treatment.
As a first step of fractionation, a freeze-thaw treatment was applied in fraction PK, to give cold-water soluble (SPK) and insoluble (PPK) fractions. In this procedure, the sample was frozen and then thaw at room temperature followed by centrifugation (8000 rpm, 15 min at 15 °C).
Fraction SPK was further fractionated by Fehling’s treatment. Briefly, it was dissolved in distilled water and treated with Fehling’s solutions [10] resulting, after centrifugation (8000 rpm, 15 min at 15 °C), in a xyloglucan-copper complex as the pellet (fraction PF-SPK) and a soluble fraction (SF-SPK). After neutralization with HOAc, both fractions were dialyzed against tap water and deionized with cation exchange resin. The fraction PF-SPK was later purified by ultrafiltration through a membrane with cut-off of 100 kDa (PLHK04710-Ultracel, Millipore), yielding the fractions PF-SPK-100E (eluted in 100 kDa) and PF-SPK-100R (retained in 100 kDa) (Figure 1).
Fraction PF-SPK-100R was further purified by anion exchange chromatography. It was dissolved in distilled water (50 mg/mL), centrifuged (12000 x g, 10 min at 10 ºC) and the supernatant applied to a DEAE-Sepharose Fast Flow column (3.0 cm×25 cm). The column was eluted with distilled water (F1) followed by 4.0 M NaCl solution (F2) at a flow rate of 1.5 mL/min. Polysaccharides in the eluted fractions were detected using phenol–sulfuric acid method [11] . The obtained fractions were concentrated and freeze-dried.
The yields were expressed as % based on the weight of dried prunes pulp that was submitted to extraction (1400 g) (Figure 1).
Sugar composition
Polysaccharides’ neutral monosaccharides composition was determined by hydrolysis with 2 M TFA (8 h/100 °C), conversion into alditol acetates using successive NaBH4 reductions, and acetylation with Ac2O-pyridine (1:1, v/v, 2 mL - 100 °C, 30min). A Varian gas chromatograph and mass spectrometer (Saturn 2000R), with He as carrier gas were used for analysis. For quantitative analysis, a capillary column (30 m x 0.25 mm i.d.) of DB-225 was held at 50 ºC during injection for 1 min, then programmed at 40 ºC/min to 220 ºC and held at this for 19.75 min.
The determination of uronic acid contents was conducted according to the m-hydroxybiphenyl method [12].
Determination of homogeneity and molecular weight of polysaccharides
The homogeneity of polysaccharides was evaluated by high performance steric exclusion chromatography (HPSEC), with a Waters 2410 differential refractometer as equipment for detection. A series of four columns, with exclusion sizes of 7 x 106 Da (Ultrahydrogel 2000, Waters), 4 x 105 Da (Ultrahydrogel 500, Waters), 8 x 104 Da (Ultrahydrogel 250, Waters) and 5 x 103 Da (Ultrahydrogel 120, Waters) was used. The eluent was 0.1 M aq. NaNO2 containing 200 ppm aq. NaN3 at 0.6 mL/min. The sample, previously filtered through a membrane (0.22 µm, Millipore), was injected (250 µl loop) at a concentration of 1 mg/mL. To obtain the molecular weight, standard dextrans (487kDa, 266kDa, 124kDa, 72.2kDa, 40.2kDa, 17.2kDa and 9.4kDa, from Sigma) were employed to obtain the calibration curve. The molecular weight of the sample was calculated according to the calibration curve.
Methylation analysis of polysaccharide
Fraction PF-SPK-100R was O-methylated as described by Ciucanu and Kerek [13]. The per-O-methylated polysaccharide was further submitted to methanolysis in 3% HCl–MeOH (80 °C, 2 h) followed by hydrolysis with H2SO4 (0.5M, 12 h) and neutralization with BaCO3. The material was then reduced an acetylated as described above for monosaccharides composition, except that NaBD4 was used for reduction. The resultant partially O-methylated alditol acetates were analyzed with a GC-MS. For separation, a 30 m x 0.25 mm i.d. capillary column of DB-225 was held at 50 ºC during injection for 1 min, then programmed at 40 ºC/min to 210 ºC and held at this temperature for 31 min. Typical electron impact breakdown profiles and retention times of partially O-methylated alditol acetates were used for identification [14].
Nuclear magnetic resonance (NMR) spectroscopy
Spectra of 13C NMR were acquired using a Bruker spectrometer (DRX 400 MHz AVANCE III NMR - Bruker Daltonics, Germany). Samples were dissolved in D2O and placed in a 5 mm inverse gradient probe, at 70 °C for analysis. Chemical shifts were expressed as δ ppm and acetone CH3 group’s resonance was used as internal standard (δ 30.2). The spectra were handled using the Topspin® (Bruker) computer program.
Results and Discussion
In order to extract water insoluble polysaccharides from prunes, the residue of prunes’ water extraction was submitted to alkaline extraction, which resulted, after dialysis, in fraction PK. This was further fractionated through freeze-thaw treatment followed by centrifugation to give rise to a supernatant fraction (SPK) and a precipitated fraction (PPK) (Figure 1). The latter presented arabinose and glucose as main monosaccharides (Table 1), but NMR analysis was not possible due to its high insolubility in different solvents.
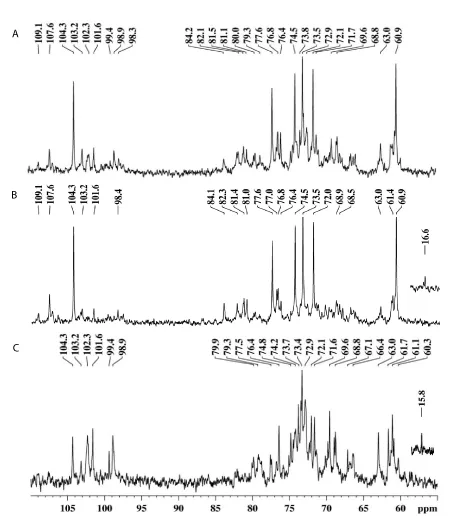
On the other hand, SPK presented arabinose and galactose as main monosaccharides (Table 1). The 13C-NMR spectrum (Figure 2A) had signals of β-D-Galp at δ 104.3 (C-1), δ 74.5 (C-5), δ 73.5 (C-3), δ 72.1 (C-2) and δ 77.6 (substituted C-4) [15-17]. The anomeric signal at δ 107.6 was assigned to units of α-L-Araf [17]. These data could indicate the presence of a type I arabinogalactan (AG-I) in fraction SPK, already reported for prunes’ water extract [18]. However, besides the signals of an AG-I, SPK presented a diversity of other anomeric signals in the region between δ 98.0 and δ 104.0 (Figure 2A), as well as significant content of glucose and xylose according to the monosaccharide analysis (Table 1), indicating the presence of another polysaccharide.
In order to separate the different polymers present in SPK, it was treated with Fehling’s solution, resulting in a precipitated fraction (PF-SPK) and a supernatant fraction (SF-SPK) (Figure 1). As previously observed for water extracts [18], the AG-I remained in the Fehling supernatant as could be seen in the 13C-NMR (Figure 2B) and monosaccharide analysis (Table 1), of fraction SF-SPK. The fraction precipitated with Fehling solution PF-SPK, had glucose and xylose as main monosaccharides (Table 1). Besides, anomeric signals of β-Glcp and α-Xylp could be seen at δ 102.3 and δ 99.4/98.9, respectively, in the 13C-NMR spectrum of PF-SPK [19] (Figure 2C). These data indicate that while the AG-I present in SPK remained soluble after Fehling treatment, the fraction containing xylose and glucose was precipitated.
Once PF-SPK had a heterogeneous profile in HPSEC (data not shown), it was further filtrated with 100 kDa cutoff Milipore membrane, yelding an eluted fraction PF-SPK-100E and a retained fraction PF-SPK-100R (Figure 1). Only fraction PF-SPK-100R presented a homogeneous elution profile when analysed by HPSEC (Figure 3). The calculated molecular weight was 66 kDa.
The monosaccharide composition of PF-SPK-100R showed glucose and xylose as main sugars, and minor amounts of arabinose, galactose and fucose (Table 1). Methylation data of PF-SPK-100R is presented on Table 2. The major methylated derivatives were 2,3,6-Me3-Glc-ol acetate (15%) and 2,3- Me2-Glc-ol acetate (21.6%), indicating the presence of 4-O- and 4,6-O-substituted glucose units. Terminal and 2-O-substituted xylose units were also found, according to the derivatives 2,3,4-Me3- and 3,4-Me2-Xyl-ol acetates, respectively. These data suggest the presence of a xyloglucan in fraction PF-SPK-100R. However, the presence of 2,3-Me2- and 3-Me-Xyl-ol derivatives, relative to 4-O- and 2,4-di-O-substituted xylose units were also present. This type of linkage is uncommon in xyloglucans since typically xylose units are exclusively O-2-linked. Although few exceptions could be found in the literature [20,21], it’s most likely that these 4-O and 2,4-di-O- substituted xylose arose from the concomitant presence of a heteroxylan in fraction PF-SPK-100R.
As the derivatives 3,4-Me2-xylitol acetate and 2,3-Me2-xylitol acetate have the same retention time in the conditions employed in the GC-MS, they were deuterated at C-1, so each component of the peak was detected by their fragmentation patterns. The 3,4-Me2-derived xylitol acetate gives ions of m/z 190, 130, 117 and 88, while the 2,3-Me2-derived xylitol acetate gives ions of m/z 189, 129, 118 and 87 [20]. From the intensity of each ion, it was observed a 1.0:1.4 ratio of 3,4-Me2-Xylp and 2,3-Me2-Xylp (Figure 4).
In addition to the methylated derivatives of glucose and xylose, 2,3,4,6-Me4-Gal-ol acetate relative to terminal galactose from xyloglucan branches was also present. Derivatives 2,3,4-Me3-Fuc-ol acetate, 2,3,4-Me3-Ara-ol-acetate and 2,3,5-Me3-Ara-ol-acetate, indicate the presence of terminal units of Fucp, Arap and Araf, respectively. Terminal fucose units are commonly found in xyloglucans from dicotyledonous [22]. Moreover, instead of fucose, Araf units were identified on side chains of xyloglucans from solanaceous plants [23]. Despite this, the concomitant presence of Fucp, Arap and Araf units is an unusual feature in xyloglucans. Thus, these Araf are likely to be side chains in the xylan, corroborating for the assumption that fraction PF-SPK-100R is composed by a mixture of a fucogalactoxyloglucan and a heteroxylan. The xyloglucan content in fraction PF-SPK-100R was estimated to be ∼68% based on the sum of 4-O and 4,6-O-linked glucose, terminal and 2-O-substituted xylose and terminal galactose and fucose.
The presence of xylan–xyloglucan complexes has been previously identified in the cell walls of olive pulp [24]. In our research group, a xyloglucan and an acid heteroxylan were also found together in alkaline extracts from starfruit (Averrhoa carambola L.) and separated through anion exchange chromatography (unpublished data). In fraction PF-SPK-100R however, due to the absence of uronic acid linked to the xylan backbone, no polymers were retained after anion exchange chromatography (Fraction F1) making its separation from the xyloglucan not possible (data not shown). Moreover, the presence of the xylan and xyloglucan was also observed in the fraction PF-SPK-100E (Figure 5A), which demonstrated a similar 13C-NMR spectrum as that of fraction PF-SPK-100R (Figure 5B). Attempts were also made to separate these polymers through ultrafiltration with 50, 30 and 10kDa membranes. However, all the retained and eluted fractions showed the presence of both polymers.
The 13C-NMR spectrum of PF-SPK-100R (Figure 5B) is in accordance with methylation data and presented the main signals related to the xylan and xyloglucan mixture. Anomeric signals related to (1→4) and (1,4→6)-linked β-Glcp units that form the main chain of the xyloglucan are found at δ 102.3 and δ 103.2, respectively [25,26]. The signal at δ 98.9 was assigned to anomeric carbons of terminal and 2-O-substituted α-Xylp units, while that at δ 99.4 and δ 15.8 can be assigned to C-1 and C-6 of terminal Fucp units, respectively. In addition, the signal at δ 104.4 can be assigned to C-1 of terminal β-Galp units [25]. Signals from the heteroxylan could be seen at δ 101.6 and δ 107.7 from anomeric carbons of β-Xylp and α-L-Araf units, respectively.
Overall, fraction SPK, which contains the insoluble dietary fibers of prunes was composed of a type I arabinogalactan, a fucogalactoxyloglucan and a heteroxylan. Despite being a soluble dietary fiber and solubilized with water [9], the type I arabinogalactan was also found herein in the fraction extracted with alkali. The presence of pectic polysaccharides associated with hemicelluloses and cellulose within the cell wall and that required harsher extraction conditions was also reported by Oechslin and others [27] in apple cellulosic residue. Thus, these findings suggest that prunes’ insoluble dietary fibers are composed by some pectic polysaccharides besides cellulose and hemicellulosic polymers.
Some biological activities have already been attributed to type I arabinogalactans, such as immunological [28-30] and anti-ulcer activities [9,31]. Xyloglucan from different sources were also previously shown to display biological activities such as hypolipidemic [32], anti-tumoral [33,34], immunomodulatory [35-38] and hypoglycemic [39-41]. It’s noteworthy, that some of these effects, such as hypolipidemic and hypoglycemic, were previously found in prunes, however, the responsible components were not fully resolved [8].
Conclusion
Prunes’ non-cellulosic insoluble fibers were found to contain, a pectic type I arabinogalactan, a fucogalactoxyloglucan and a heteroxylan, suggesting that IDF can be composed by some pectic polysaccharides besides cellulose and hemicellulosic polymers. Moreover, this paper brings important structural features of insoluble dietary fibers from prunes that may be associated to biological functions, and provides new insights into the diversity of fruit hemicellulosic polymers.
This research was supported by Projeto Universal (Process 477971/2012-1) provided by CNPq foundation (Brazil) and by PRONEX-Carboidratos. The author Thaisa Moro Cantu-Jungles received a fellowship from CNPq foundation. The authors are grateful to the NMR Center of UFPR for recording the NMR spectra.
- Codex Alimentarius (2008) Report of the 30th session of the codex committee on nutrition and foods for special dietary uses, Cape Town, South Africa, 3–7 November 2008.
- Dietary Fiber Definition Committee, DFD (2001) Report of the Dietary Fiber Definition Committee to the Board of Directors of the American Association of Cereal Chemists: The Definition of Dietary Fiber, in St Paul, MN: AACC International: 112-126.
- Verspreet J, Damen B, Broekaert WF, Verbeke K, Delcour JA, et al. (2016) A Critical Look at Prebiotics Within the Dietary Fiber Concept. Annual review of food science and technology 7: 167-190. Link: https://goo.gl/l0QnZ2
- Fuller S, Beck E, Salman Hand, Tapsell L (2016) New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods for Human Nutrition 71: 1-12. Link: https://goo.gl/56dN5s
- Kleessen B, Sykura B, Zunft H-J, Blaut M (1997) Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. American Journal of Clinical Nutrition 65: 1397-1402. Link: https://goo.gl/ccuQwh
- Hamaker BR, Tuncil YE (2014) A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. Journal of molecular biology 426: 3838-3850. Link: https://goo.gl/D7V3RY
- Liu J, Willför S, Xu C (2015) A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioactive Carbohydrates and Dietary Fibre 5: 31-61. Link: https://goo.gl/J8XEbs
- Stacewicz-Sapuntzakis M, Bowen PE, Hussain EA, Damayanti-Wood BI, Farnsworth NR (2001) Chemical composition and potential health effects of prunes: a functional food? Critical reviews in food science and nutrition 41: 251-286. Link: https://goo.gl/eHVes9
- Cantu-Jungles TM, Maria-Ferreira D, da Silva LM, Baggio CH, Werner MF, et al. (2014) Polysaccharides from prunes: Gastroprotective activity and structural elucidation of bioactive pectins. Food chemistry 146: 492-499. Link: https://goo.gl/9iytqK
- Jones J, Stoodley R (1965) Fractionation using copper complexes. Methods in Carbohydrate Chemistry 5: 36-38.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350-356. Link: https://goo.gl/h7AnoY
- Filisetti-Cozzi TM, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry 197: 157-162. Link: https://goo.gl/VBsHKz
- Ciucanu I, Kerek F (1984) Simple and rapid method for permethylation of carbohydrates. Carbohydrate Research 131: 209-217. Link: https://goo.gl/1Cq30x
- Sassaki GL, Gorin PA, Souza LM, Czelusniak PA, Iacomini M (2005) Rapid synthesis of partially O-methylated alditol acetate standards for GC–MS: Some relative activities of hydroxyl groups of methyl glycopyranosides on Purdie methylation. Carbohydrate Research 340: 731-739. Link: https://goo.gl/l1R0iy
- Gorin PAJ, Mazurek M (1975) Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Canadian Journal of Chemistry 53: 1212-1223. Link: https://goo.gl/xClI6p
- Tischer CA, Gorin PAJ, Iacomini M (2002) The free reducing oligosaccharides of gum arabic: aids for structural assignments in the polysaccharide. Carbohydrate Polymers 47: 151-158. Link: https://goo.gl/6VW3jZ
- Delgobo CL, Gorin PA, Tischer CA, Iacomini M (1999) The free reducing oligosaccharides of angico branco (Anadenanthera colubrina) gum exudate: an aid for structural assignments in the heteropolysaccharide. Carbohydrate Research 320: 167-175. Link: https://goo.gl/RpkHZ3
- Cantu-Jungles TM, Maria-Ferreira D, da Silva LM, Baggio CH, Werner MF, et al. (2014) Polysaccharides from prunes: Gastroprotective activity and structural elucidation of bioactive pectins. Food chemistry 146: 492-499. Link: https://goo.gl/TLF4FS
- Busato AP, Vargas-Rechia CGand, Reicher F (2001) Xyloglucan from the leaves of Hymenaea courbaril. Phytochemistry 58: 525-531. Link: https://goo.gl/2NjTVw
- Busato A, Vargas-Rechia C, Gorin P, Petkowicz C, Tischer C, et al. (2005) New 4-O-substituted xylosyl units in the xyloglucan from leaves of Hymenaea courbaril. International journal of biological macromolecules 35: 277-282. Link: https://goo.gl/e26COe
- Peña MJ, Darvill AG, Eberhard S, York WSand, O’Neill MA (2008) Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants*. Glycobiology 18: 891-904. Link: https://goo.gl/WUcTw1
- Hayashi, T (1989) Xyloglucans in the primary cell wall. Annual Review of Plant Physiology and Plant Molecular Biology 40: 139-168. Link: https://goo.gl/DU3vTV
- York WS, Kolli VK, Orlando R, Albersheim P, Darvill AG (1996) The structures of arabinoxyloglucans produced by solanaceous plants. Carbohydrate Research 285: 99-128. Link: https://goo.gl/ERd7Og
- Coimbra MA, Rigby NM, Selvendran RR, Waldron KW (1995) Investigation of the occurrence of xylan-xyloglucan complexes in the cell walls of olive pulp (Olea europaea). Carbohydrate polymers 27: 277-284. Link: https://goo.gl/zvEoOk
- Busato AP, Vargas-Rechia CG, Reicher F (2001) Xyloglucan from the leaves of Hymenaea courbaril. Phytochemistry 58: 525-531. Link: https://goo.gl/VfZ2gZ
- Hantus S, Pauly M, Darvill AG, Albersheim P, York WS (1997) Structural characterization of novel L-galactose-containing oligosaccharide subunits of jojoba seed xyloglucans. Carbohydrate research 304: 11-20. Link: https://goo.gl/dZtIrz
- Oechslin R, Lutz MV, Amadò R (2003) Pectic substances isolated from apple cellulosic residue: structural characterisation of a new type of rhamnogalacturonan I. Carbohydrate Polymers 51: 301-310. Link: https://goo.gl/kC5QVF
- Iacomini M, Serrato RV, Sassaki GL, Lopes L, Buchi DF, et al. (2005) Isolation and partial characterization of a pectic polysaccharide from the fruit pulp of Spondias cytherea and its effect on peritoneal macrophage activation. Fitoterapia 76: 676-683. Link: https://goo.gl/RtY112
- Inngjerdingen KT, Kiyohara H, Matsumoto T, Petersen D, Michaelsen TE, et al. (2007) An immunomodulating pectic polymer from Glinus oppositifolius. Phytochemistry 68: 1046-1058. Link: https://goo.gl/rCo7c8
- Nergard CS, Diallo D, Inngjerdingen K, Michaelsen TE, Matsumoto T, et al. (2005) Medicinal use of Cochlospermum tinctorium in Mali: anti-ulcer-, radical scavenging-and immunomodulating activities of polymers in the aqueous extract of the roots. J Ethnopharmacol. 96: 255-269. Link: https://goo.gl/Na6Icp
- Cipriani TR, Mellinger CG, Bertolini ML, Baggio CH, Freitas CS, et al. (2009) Gastroprotective effect of a type I arabinogalactan from soybean meal. Food Chemistry 115: 687-690. Link: https://goo.gl/CA3sD8
- Yamatoya K, Shirakawa M, Kuwano K, Suzuki Junko, Mitamura T (1996) Effects of hydrolyzed xyloglucan on lipid metabolism in rats. Food hydrocolloids 10: 369-372. Link: https://goo.gl/XyIzJs
- Kato Y, Uchida J, Ito, Yasushi Mitsuishi (2001) Structural analysis of the oligosaccharide units of xyloglucan and their effects on growth of COLO 201 human tumor cells. International Congress Series. 1223:161–164. Link: https://goo.gl/oAAGqA
- Hensel A, Meier K (1999) Pectins and xyloglucans exhibit antimutagenic activities against nitroaromatic compounds. Planta Med. 65: 395-399. Link: https://goo.gl/c8Np38
- Rosário M, Noleto G, Bento J, Reicher F, Oliveira M, et al. (2008) Effect of storage xyloglucans on peritoneal macrophages. Phytochemistry 69: 464-472. Link: https://goo.gl/GHXlqF
- Pauly M, Andersen LN, Kauppinen S, Kofod LV, York, WS, et al. (1999) A xyloglucan-specific endo-β-1, 4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiology 9: 93-100. Link: https://goo.gl/dmtvli
- Puhlmann J, Zenk Mand, Wagnert H (1991) Immunologically active polysaccharides of Arnica montana cell cultures. Phytochemistry 30: 1141-1145. Link: https://goo.gl/oumSTq
- Wagner, H, Stuppner, H, Schäfer, Wand Zenk, M (1988) Immunologically active polysaccharides of Echinacea purpurea cell cultures. Phytochemistry 27: 119-126. Link: https://goo.gl/Hj5Rzg
- Maiti, R, Jana, D, Das, Uand Ghosh, D (2004) Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J Ethnopharmacol. 92: 85-91. Link: https://goo.gl/cDfCMk
- Sone, Y, Makino, Cand Misaki, A (1992) Inhibitory effect of oligosaccharides derived from plant xyloglucan on intestinal glucose absorption in rat. Journal of nutritional science and vitaminology 38: 391-395. Link: https://goo.gl/DHO7XC
- Martinello, F, Soares, S, Franco, J, Santos, A, Sugohara, A, et al. (2006) Hypolipemic and antioxidant activities from Tamarindus indica L. pulp fruit extract in hypercholesterolemic hamsters. Food Chem Toxicol 44: 810-818. Link: https://goo.gl/xmojZ2
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
