Journal of Food Science and Nutrition Therapy
Serum levels of Vitamin A and Atopic Rhinoconjunctivitis in Swedish adolescents
Malin Barman1,2*, Nils-Gunnar Carlsson1, Anna Sandin3, Agnes E. Wold4 and Ann-Sofie Sandberg1
• Previous studies show contradictory results on the relevance of vitamin A status in clinical allergy
. • We found no clear association between serum levels of vitamin A and atopic rhinoconjunctivitis in Swedish teenagers.
Cite this as
Barman M, Carlsson NG, Sandin A, Wold AE, Sandberg AS (2017) Serum levels of Vitamin A and Atopic Rhinoconjunctivitis in Swedish adolescents. J Food Sci Nutr The 3(1): 014-019. DOI: 10.17352/jfsnt.000009Aim: Vitamin A plays a role in mucosal immunity and tolerance, but the association between vitamin A status and allergy is still unclear. The aim of the study was to analyze the levels of vitamin A in serum from adolescents with or without atopic rhinoconjunctivitis.
Method: Thirteen-year-old children with atopic rhinoconjunctivitis (n = 53) and non-allergic, non-sensitized controls (n = 52) were randomly selected from a population based prospective birth cohort comprising 1228 children in Northern Sweden born in 1996-1997. Vitamin A (retinol) concentrations in serum were measured with high performance liquid chromatography mass spectrometry. Multiple logistic regression was used to evaluate the association between allergy prevalence and serum vitamin A levels.
Results: Multiple logistic regression analysis showed no association between serum vitamin A levels and atopic rhinoconjunctivitis prevalence; OR = 1.00 (95% confidence interval 1.00-1.00), p = 0.81. Stratification for gender revealed a trend for a higher risk for having atopic rhinoconjunctivitis with higher concentrations of vitamin A in serum for females, OR = 1.02 (1.00-1.05), p = 0.07. No such associations were found in male subjects OR = 0.99 (0.97-1.01), p = 0.15. A dose-response relationship between allergy and vitamin A concentrations were also calculated but no such relationships were found, neither for all subjects nor for male and females separately.
Conclusions: Serum levels of vitamin A could neither be positively nor negatively associated with atopic rhinoconjunctivitis in Swedish teenagers.
Introduction
Allergy is the most prevalent chronic disease in affluent countries with a Western lifestyle, and its prevalence has increased globally. Decreased exposure to environmental microbes [1,2], and changes in dietary patterns, including an increased intake of margarine and oils [3], are different factors that may explain the rise in the prevalence of allergy.
Vitamin A (retinol) is a fat-soluble vitamin that is important for vision, gene expression and tissue differentiation. Vitamin A deficiency has profound effects on the immune system [4], and is associated with increased mortality from infectious disease in developing countries [5]. Animal studies show that vitamin A-deficient animals have reduced mucosal immune responses [6], and a reduced capacity to develop oral tolerance [7]. A particular subset of antigen-presenting dendritic cells (DC), the CD103+DC subset, possesses the vitamin A converting enzyme RALDH [8], and is central in mucosal immunity and tolerance [7]. These cells traffic the intestinal mucosa, where they pick up antigens, migrate to the mesenteric lymph nodes and present the antigens to naïve T cells trafficking the lymph nodes. Retinoic acid (a metabolite of vitamin A) produced by the CD103+DCs is needed to imprint a gut-migrating phenotype on the naïve T cells [8], and to convert naïve T cells to regulatory T cells [9]. Retinoic acid has been shown both to promote Th2 cell differentiation by increasing the ratio of Th2 cytokines relative to Th1 cytokines [10], and to downregulate Th2 immune responses by inhibiting IL-6 driven induction of Th17 cells and increasing the proliferation of T-regulatory cells [11,12].
The importance of retinoic acid for mucosal immunity and oral tolerance and the effect on T-regulatory cells implies that the availability of vitamin A could have an impact on allergy development. A systematic review from 2009 on dietary intake and serum levels of vitamin A metabolites and asthma found serum levels of retinol to be inversely associated with asthma in children [13]. Similar results were reported in another meta-analysis from 2010, which revealed lower serum levels of vitamin A in children with asthma compared with controls [14].
The aim of the present study was to analyze the association between vitamin A in serum and allergy in Swedish adolescents selected from a prospective and population based mother and child birth cohort. Allergy has many diagnoses and nuances with different, and sometimes transient, age influenced symptoms in combination with environmental changes, and is best observed in longitudinally prospective cohorts. In the present study, we had the opportunity to select non-allergic children with no sensitization or reported allergic symptoms neither at 1, 4 nor 13 years of age. For a well-defined group of children with allergy at 13 years of age (time point for retinol analyses) we chose to study allergic rhinoconjunctivitis, defined as coherently reported symptoms and sensitization to airborne allergens.
Methods
Study subjects
Cases with atopic rhinoconjunctivitis (n = 53) and non-sensitized non-allergic controls (n = 52) were selected at 13 years of age from a population based birth cohort consisting of 1,228 children born during one year between February 1996 and January 1997 at the Östersund Hospital in Northern Sweden [15]. The children were followed with skin prick tests and questionnaires regarding allergic symptoms at 1, 4 and 13 years of age. At 13 years of age, 789 adolescents responded to the questionnaire (together with a parent) and participated in sensitization test. The subjects were skin prick tested with standardized extracts for ten allergens, including milk, egg, fish, wheat, soy, cat, dog, horse, timothy grass, and birch (ALK, Hørsholm, Denmark), as earlier described [16,17].
Allergy diagnosis
Atopic rhinoconjunctivitis was defined as a positive skin prick test to one or more inhalant allergens (cat, dog, horse, birch or timothy), in combination with a positive answer to the question “Have you had any allergic symptoms from eyes and/or nose in contact with pollen or furred animals during the last 12 months?”. Hence, all subjects in the allergic group had symptoms in contact with pollen or furred animals in combination with a positive skin prick test against an airborne allergen. In total, 174 of the subjects in the cohort fulfilled the criteria for atopic rhinoconjunctivitis. Non-allergic, non-sensitised controls were defined as having neither allergic symptoms nor any positive reaction in the skin prick test at 13 years of age or in any of the previous follow-ups (n = 331).
Selection of subjects
Fifty-three subjects who had atopic rhinoconjunctivitis were choosen randomly; of these, 19 subjects also had asthma, 4 subjects also had food allergy and 30 subjects had only rhinoconjunctivitis. As controls we selected a similar number of subjects that had been non-allergic and non-sensitised in all follow-ups at 1, 4, and 13 years of age.
Collection of serum
Venous blood (10 mL) was drawn, allowed to clot and centrifuged. Serum was separated, aliquoted and frozen within three hours. The serum samples were stored for a maximum of five years at -80 oC before analyses.
Analysis of vitamin A in serum
Serum vitamin A concentrations were measured using liquid chromatography mass spectrometry (LC-MS) simultaneously as 25-hydroxy vitamin D. The results from the vitamin D analysis has been reported previously [18]. Samples were prepared for the analysis according to Turpeinen et al. [19], with some modifications as earlier described in detail [18]. Briefly, 200 µl serum was mixed with 150 µl methanol:iso-propanol (80:20), containing 100 ng of retinoic acid as internal standard (Sigma Aldrich, Saint Louis, USA). After extraction twice with 2 ml hexane, the organic phases were evaporated and dissolved in 100 µl of methanol and transferred to vials. Retinol and retinoic acid were analyzed using an LC-MS system (Agilent 1260 Infinity Binary LC and Agilent 6120 Quadrupole LC/MS, Agilent Technologies, Santa Clara, California, USA). The LC-MS setup has previously been described in detail [18]. The instrument was operating in selected-ion monitoring mode: 269 for both retinol and retinoic acid. Retinol and retinoic acid were identified based on retention time.
A control serum sample was extracted and analyzed in every run. Study samples and control samples were prepared and analyzed in duplicates. Relative standard deviation (%RSD) for intra-assay precision was 4.7% for a serum sample with a mean retinol concentration of 116 µMol/L. Relative standard deviation was 7.5% for between day variability. Retinol concentrations decreased from 116 µMol/L to 111 µMol/L after 24 hours in room temperature. After three freeze-thaw cycles, i.e. thawed in room temperature and frozen for 24 hours, retinol concentration was down to 99 µMol/L.
Statistical analysis
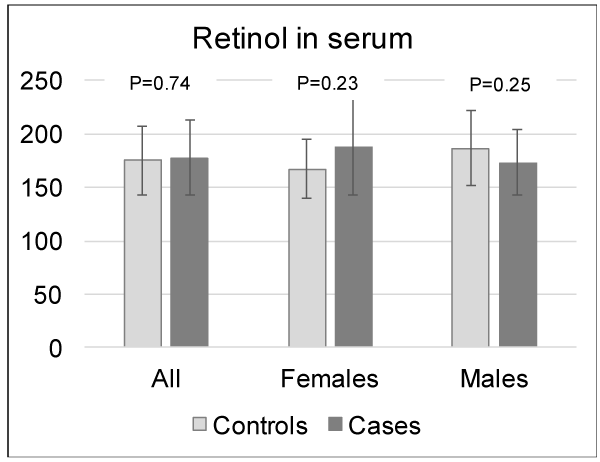
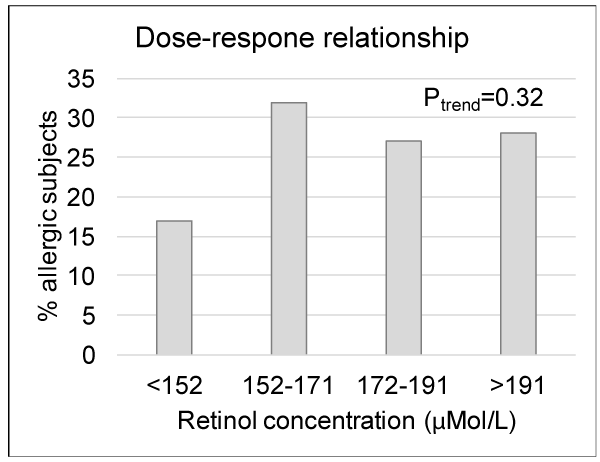
For analysis of differences in background variables (Table 1), chi-square test was used. Mann-Whitney U test was used to compare group means of serum vitamin A concentrations due to non-parametric data (Figure 1). To analyse the trend for dose-response in figure 2 chi-square for linearity was used. Multiple logistic regression analysis was used to analyze the association between allergy and serum vitamin A levels, taking the following potential confounders into consideration: gender, siblings, breastfeeding, maternal allergy, paternal allergy and residence of school (Table 2). Statistical analyses were performed using IBM SPSS Statistics version 19 (IBM Corporation, New York) and a two-tailed p-value ≤0.05 was considered significant.
Ethical considerations
The study was approved by the local ethical committee in Umeå, Sweden (Dnr 09-110M) and was conducted according to the Declaration of Helsinki. At 13 years of age, the adolescents verbally approved their participation in skin prick tests and collection and analysis of blood samples for vitamin A, and their parents provided written consent on behalf of the children. Participation was voluntary and the adolescents were informed that they were free to decline participation at any time, without stating the reason and that their decision would not lead to any disadvantage.
Results
Characteristics
The characteristics of the individuals selected from each group as well as the non-selected individuals, are shown in Table 1. Statistical analyses were performed to compare both selected with non-selected subjects as well as to compare cases and controls. For differences between selected and non-selected subjects in the two groups, a larger percentage of selected non-allergic adolescents attended schools in the only major city of the region, Östersund, compared to the non-selected non-allergic adolescents (Table 1). No significant differences were found between selected and non-selected cases. For the comparisons between cases and controls: subjects with atopic rhinoconjunctivitis were more often males and had more often mothers with a history of allergy than selected non-allergic controlsA;also paternal allergic heredity tended to be higher in cases than in controls (Table 1).
Differences in serum vitamin A concentrations
Vitamin A was extracted and quantified in serum samples of 105 individuals representing the cases (n = 53) and controls (n = 52). The mean (SD) serum concentration of vitamin A was 176 (34) µMol/L for the group as a whole. No significant differences in vitamin A serum concentrations were noted between cases and controls, 177 (35) µMol/L vs 175 (32) µMol/L respectively, p>0.2, (Figure 1). As the distribution of males and females were unevenly distributed among the cases and controls the analysis was also performed for males and females separately; the mean serum concentration of vitamin A in serum was 178 ± 33 µMol/L for males and 173 ± 35 for females (p = 0.26). No significant differences in vitamin A serum concentrations were noted between cases and controls for neither female nor males (Figure 1).
Multiple logistic regressions
Multiple logistic regression was used to analyse the association between serum vitamin A concentration and allergy diagnosis at age 13, either as crude odds ratio, or adjusted for gender, siblings, breastfeeding, maternal allergy, paternal allergy and residence of school. Serum levels of vitamin A was not a risk factor for being allergic at 13 years of age in neither the crude nor the adjusted model (Table 2). The logistic regression models were also performed separately for males and females. For females, there was a tendency for a higher risk for atopic rhinoconjunctivitis for higher vitamin A levels in serum. This association was not found for male subjects (Table 2).
Of the 53 selected subjects with atopic rhinoconjunctivitis, 19 subjects had both asthma and rhinoconjunctivitis. The logistic regression analyses were performed stratified for ‘having asthma’ and ‘not having asthma’. No significant difference in serum vitamin A levels was found between cases with or without asthma and controls (data not shown).
Dose-response relationship
To analyze if there was a dose-dependent difference between vitamin A and allergy, all subjects were divided in four groups depending on vitamin A status. Proportions of allergic subjects in each vitamin A group are displayed in Figure 2. We found no significant difference in prevalence of allergy between the four groups stratified according to serum vitamin A concentration (Chi-square for linearity, Figure 2).
Discussion
Serum levels of vitamin A were measured in 105 adolescents at the age of 13 years, living in the County of Jämtland in Northern Sweden. The subjects were followed from birth onwards with regular skin prick tests and questionnaires probing for allergic symptoms. From a well-defined group of children with allergy diagnosis based on questionnaire and skin prick test, we randomly selected cases with atopic rhinoconjunctivitis, i.e. subjects with a positive prick test to one or more airborne allergen (cat, dog, horse, birch or timothy) together with symptoms from the eye or nose in contact with furred animals or pollen. Moreover, we randomly selected non-sensitized, non-allergic controls, i.e. subjects without sensitization and symptoms at 13 years of age or in any of the previous follow-ups. No differences in serum vitamin A levels were found between adolescents with atopic rhinoconjunctivitis and healthy controls of the same age, in neither the crude nor the adjusted logistic regression model. Since the distribution of the two genders were different in the cases and the controls, with more males in the cases than in the controls, the logistic regression models were also performed separately for females and males. For females only, vitamin A tended to be associated with an increased risk for having atopic rhinoconjunctivitis. This was not shown for males separately. Hence, it seems likely that there might be a gender difference in the association between vitamin A and allergy. When the mean values of vitamin A in cases and controls was visualized with a bar chart, a slightly higher mean vitamin A level were seen in female cases than in female controls, however this difference was not statistically significant.
Previous studies on vitamin A in serum and allergy have shown mainly inverse associations [13,14,20-24], but also no associations [25-29], between vitamin A in serum and asthma. The findings of low levels of vitamin A in subjects with asthma may either be due to a casual effect of vitamin A on asthma or simply be a consequence of the disease. Factors involved in asthma exacerbations, such as airway inflammation and pulmonary infections, have been shown to increase cellular demand or urinary excretion of vitamin A and hence result in decreased serum concentrations of vitamin A.
Most of the previous studies have been focusing on asthma which has mainly been diagnosed as recurrent cough and/or wheezing, sometimes together with previous demonstration of improvement of symptoms in response to asthma medication. In young children, asthma is often diagnosed as wheezing, which may also be a sign of respiratory infection. Furthermore, asthma symptoms in adults are often triggered by viral infections. As this birth cohort used questionnaires for symptoms and did not have any objective method i.e. lung function test, we considered the diagnostic tools not sharp enough for a clear allergic asthma diagnose. Instead we chose to study allergic rhinoconjunctivitis, defined as coherently reported symptoms and sensitization to airborne allergens. Allergy is defined as symptoms in contact with the allergen and no symptoms when the allergen is removed. Sensitization to airborne allergens is an important piece of the puzzle and will further strengthen an allergy diagnose. Rhinoconjunctivitis could be well diagnosed from skin prick tests and questionnaires in this study, since parents to children at this age know for sure if the children have allergic reactions in contact with cat, dog or horse or if the children get allergic symptoms during some part of the summer (allergic reactions to i.e. pollen).
We have previously shown that neither serum levels of 25-hydroxy vitamin D nor proportions of long chain polyunsaturated fatty acids (LCPUFAs) in serum were associated with allergy in the same study population cohort in a cross-sectional analysis of the same cohort at 13 years of age [17,18]. On the other hand, we found that higher proportions of LCPUFAs in the cord blood of both the n-3 and n-6 families, were associated with being allergic at 13 years of age in this cohort [16]. This suggests that perinatal exposure to fatty acids and perhaps other factors, such as fat-soluble vitamins, may influence immune maturation and thereby subsequent allergy development. Unfortunately, we had no possibility to analyze vitamin A in the cord serum samples due to low amount of remaining cord serum.
A few studies have assessed the relation between early life exposure to vitamin A and allergy later in life. A randomized clinical trial in a vitamin A-deficient population in Nepal found no relationship between supplementation with vitamin A early in life and child asthma at nine to 23 years of life in more than 5000 subjects [30]. An observational study of 200 subjects analyzed vitamin A levels in serum at birth at two, four and twelve months of age, and at five and elven years of age. Allergy was assessed at five, eleven and 20 years of age [22]. Overall, the study found no association between serum vitamin A levels and atopic disease. The only significant relation was an inverse association between vitamin A status at 2 months of age and atopic allergy at 20 years of age [22]. In a recent study, serum carotenoids analysed at various time points during early childhood were related to parental reported asthma at five years of age [31]. Carotenoids have antioxidant capacity and are also precursors for vitamin A. The study found no associations between serum carotenoids early in life and the risk of asthma at five years of age [31].
Conclusion
We found no association between levels of vitamin A in serum and atopic rhinoconjunctivitis at 13 years of age in adolescents, selected from a population-based cohort followed from birth onwards and with well-defined allergic manifestations.
We thank Otto Savolinen for technical assistance and Anna Bernholm for blood sampling and performing skin prick tests.
- Wold AE (1998) The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53(46 Suppl): 20-25. Link: https://goo.gl/MU5Z8m
- Strachan D (1989) Hay fever, hygiene, and household size. British Medical Journal 299: 1259 - 1260. Link: https://goo.gl/tS8dFT
- Sausenthaler S, Koletzko B, Heinrich J (2006) Dietary fat intake and allergic diseases. Curr Nutr Food Sci 2: 351-359. Link: https://goo.gl/pMPnho
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8: 685-698. Link: https://goo.gl/xH14H2
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, et al. (1986) Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet (London, England).1: 1169-1173. Link: https://goo.gl/xCWwrU
- Wiedermann U, Hanson LA, Holmgren J, Kahu H, Dahlgren UI (1993) Impaired mucosal antibody response to cholera toxin in vitamin A-deficient rats immunized with oral cholera vaccine. Infection and immunity. 61: 3952-3957. Link: https://goo.gl/KSTnCJ
- Bjersing JL, Telemo E, Dahlgren U, Hanson LA (2002) Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer's patches of vitamin A deficient rats. Clinical and experimental immunology. 130: 404-408. Link: https://goo.gl/Xju22B
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, et al. (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21: 527-538. Link: https://goo.gl/ym1vvp
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ (2007) All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. The Journal of experimental medicine 204: 1765-1774. Link: https://goo.gl/7xg9wU
- Ross AC (2012) Vitamin A and retinoic acid in T cell-related immunity. The American journal of clinical nutrition 96: 1166S-1172S. Link: https://goo.gl/BL2365
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ (2007) All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204: 1765-1774. Link: https://goo.gl/S3CwSp
- Mucida D1, Park Y, Kim G, Turovskaya O, Scott I, et al. (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256-260. Link: https://goo.gl/Q5X4Wm
- Allen S, Britton JR, Leonardi-Bee JA (2009) Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax 64: 610-619. Link: https://goo.gl/wtSNi5
- Nurmatov U, Devereux G, Sheikh A (2010) Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. Journal of Allergy and Clinical Immunology 127: 724-733.e30. Link: https://goo.gl/FRtgwx
- Sandin A, Bjorksten B, Braback L (2004) Development of atopy and wheezing symptoms in relation to heredity and early pet keeping in a Swedish birth cohort. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 15: 316-322. Link: https://goo.gl/7rUc9Y
- Barman M, Johansson S, Hesselmar B, Wold AE, Sandberg A-S, et al. (2013) High Levels of Both n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids in Cord Serum Phospholipids Predict Allergy Development. PloS one 8: e67920. Link: https://goo.gl/7SCM2i
- Barman M, Jonsson K, Sandin A, Wold AE, Sandberg AS (2014) Serum fatty acid profile does not reflect seafood intake in adolescents with atopic eczema. Acta paediatrica. 103: 968-976. Link: https://goo.gl/jLmQTH
- Barman M, Jonsson K, Hesselmar B, Sandin A, Sandberg AS, et al. (2015) No association between allergy and current 25-hydroxy vitamin D in serum or vitamin D intake. Acta paediatrica 104: 405-413. Link: https://goo.gl/9WCNBc
- Turpeinen U, Hohenthal U, Stenman U-H (2003) Determination of 25-Hydroxyvitamin D in Serum by HPLC and Immunoassay. Clin Chem 49: 1521-1524. Link: https://goo.gl/mfK2xM
- Al Senaidy AM (2009) Serum vitamin A and beta-carotene levels in children with asthma. The Journal of asthma : official journal of the Association for the Care of Asthma 46: 699-702. Link: https://goo.gl/3iATP7
- Arora P, Kumar V, Batra S (2002) Vitamin A status in children with asthma. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 13: 223-226. Link: https://goo.gl/pTsX4e
- Pesonen M, Kallio MJT, Siimes MA, Ranki A (2007) Retinol concentrations after birth are inversely associated with atopic manifestations in children and young adults. Clinical and Experimental Allergy. 37: 54-61. Link: https://goo.gl/ZE9SuS
- Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Della Vecchia R, et al. (2007) Plasma lycopene and antioxidant vitamins in asthma: the PLAVA study. The Journal of asthma: official journal of the Association for the Care of Asthma. 44: 429-432. Link: https://goo.gl/824jUJ
- Mizuno Y, Furusho T, Yoshida A, Nakamura H, Matsuura T, et al. (2006) Serum vitamin A concentrations in asthmatic children in Japan. Pediatrics international: official journal of the Japan Pediatric Society 48: 261-264. Link: https://goo.gl/Ys6GNj
- Harik-Khan RI, Muller DC, Wise RA (2004) Serum vitamin levels and the risk of asthma in children. American journal of epidemiology. 159: 351-357. Link: https://goo.gl/zGzfZs
- Powell CV, Nash AA, Powers HJ, Primhak RA (1994) Antioxidant status in asthma. Pediatric pulmonology 18:34-38. Link: https://goo.gl/WcmuBd
- Baker JC, Tunnicliffe WS, Duncanson RC, Ayres JG (1999) Dietary antioxidants and magnesium in type 1 brittle asthma: a case control study. Thorax 54: 115-118. Link: https://goo.gl/gFo66g
- Picado C, Deulofeu R, Lleonart R, Agusti M, Mullol J, et al. (2001) Dietary micronutrients/antioxidants and their relationship with bronchial asthma severity. Allergy 56: 43-49. Link: https://goo.gl/eADWFo
- Elenius V, Palomares O, Waris M, Turunen R, Puhakka T, et al. (2017) The relationship of serum vitamins A, D, E and LL-37 levels with allergic status, tonsillar virus detection and immune response. PloS one 12: e0172350. Link: https://goo.gl/KXDzuB
- Checkley W, West KP, Wise RA, Wu L, LeClerq SC, et al. (2011) Supplementation with vitamin A early in life and subsequent risk of asthma. European Respiratory Journal 38: 1310-1319. Link: https://goo.gl/nim4MY
- Hamalainen N, Nwaru BI, Erlund I, Takkinen HM, Ahonen S, et al. (2017) Serum carotenoid and tocopherol concentrations and risk of asthma in childhood: a nested case-control study. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 47: 401-409. Link: https://goo.gl/VjHiBx
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
