Journal of Food Science and Nutrition Therapy
Variability of physio-chemical, anti-oxidant, and sensorial quality of newly released papaya varieties in Ethiopia
Masresha Minuye1* and Mulate Zerihune2
2Ethiopian Institute of Agricultural Research, EIAR, P.O. Box 2003, Addis Ababa, Ethiopia
Cite this as
Minuye M, Zerihune M (2020) Variability of physio-chemical, anti-oxidant, and sensorial quality of newly released papaya varieties in Ethiopia. J Food Sci Nutr The 6(1): 015-021. DOI: 10.17352/jfsnt.000021A study was carried out to investigate the physic-chemical, proximate, antioxidant, and sensorial qualities of the released papaya varieties namely, KK-103, MK-121, and CMF-078 which are widely grown in Ethiopia. Results showed that maximum in fruit physical parameters was observed for variety MK-121 and the lowest value was for CMF-078. Results of TSS, citric acid, total carotenoid, beta-carotene, and vitamin C content of the three papaya fruits were covered in the range of 10.287-12.620 (Brix), 1.455- 1.978(g/l), 12.564- 17.860 (µg/g), 2.131- 3.036 (µg/g) and 30.854-43.407 (mg /100g) respectively. The proximate compositions of papaya varieties were also analyzed and little differences were observed at p<0.05. Besides, the sensory evaluation was carried out and the result showed that in variety MK-121 exhibited significantly higher values for color, flavor, sourness, and sweetness than did the other two varieties. However, the results of the sensory evaluation showed that variety CMF-078 had higher acceptability than did the other two varieties. Therefore, the author recommended that fruits of variety MK-121 could be better for fresh consumption as well as for processing purposes to enrich the food products with anti-oxidants.
Introduction
Papaya (Carica papaya L.) is one of the important and versatile fruits and grown worldwide in the tropics and subtropics including India, Bangladesh, Malaysia, Australia, Hawaii, Philippines, Sri Lanka, South Africa, and other countries in tropical America [1]. Papaya has been ranked as one of the tops for nutritional value among 38 common fruits [2]. It is available all around the year and consumed as fruit after ripe and green papaya as a vegetable. Besides, they have medicinal properties and have been used against diseases for many years [3,4]. Practically every part of the fruit is used for a variety of medical purposes [5]. It has been argued by scientists that all parts of papaya, including seeds, roots, rinds, and fruits have positive effects on general health preventing diseases [6].
Fruit quality is one of the most important themes of the fruit industry, especially for juice and pulp processing as they have a direct impact on the use of additive synthetic products. Quality is defined as the absence of defects or degrees of excellence which consists of appearance, color, shape, injuries, flavor, taste, aroma, nutritional value, and is safe for the consumer. Physical and chemical properties of fruits are important indicators of their maturation and also the internal and external quality that affect market demands. Due to a higher market exigency for high-quality products, the juice, and pulp industries have been looking for fruits with better internal and external features, including fruit physical, chemical, and anti-oxidant contents [7]. Moreover; papaya also the main source of many vitamins, such as vitamin C, and it contains vitamin E, pectin, and carotenoids [8]. Despite its economic importance and nutritional value, papaya fruit had grown and produced on a large scale across the world including Ethiopia.
Recently in Ethiopian three papaya varieties had been released by the Ethiopian Institute of agricultural research, Melkassa agricultural research center but their nutritional and sensorial aspect of this papaya varieties has not been well studied and documented. Hence full characterization of the quality attributes of released papaya varieties needs research attention and physic-chemical characteristics are important qualitative indexes of any fruit for fresh consumption [8]. Therefore; the present study aims was to conduct a detailed analysis and assess the variations in fruit physicochemical characteristics, nutritional and sensory evaluation of the three released papaya varieties in Ethiopia.
Materials and methods
Study areas
Studies were conducted in the Food Science and Nutrition research laboratory of Melkassa Agricultural Research Center, one of the research centers of the Ethiopian Institute of Agricultural Research. The Center is located in the Ethiopian rift valley, 117 km away from Addis Ababa in the southeast direction located at 8°24’N and 39°12’E and an altitude of 1550 m. The mean minimum and maximum temperature of the environment are 14.8 °C and 28.6 °C. The Center receives a mean of the total annual rainfall of 825.9 mm with erratic distribution, having a high coefficient of variation. The soil contains volcanic ash but is mainly sandy loam with a pH range of 6-8.
Sample collection and preparation
Papaya fruit samples were collected from eight randomly selected trees of each variety. The papaya samples were free from mechanical damage, insect infestation, disease, and physiological disorders. The three released papaya varieties (kk-103, MK-121, and CMF-078) were used for sample collection at a similar stage of maturity. The collected samples were stored at 12oC for further analysis. Fruit maturity was defined based on the skin color change which is a yellow color that does cover more than 75% of the skin surface. Methods of Complete Random Design experimental design was used for this study Figure 1.
Sample preparation
Ripe fruit samples were washed with deionized water to remove dust particles and pathogens from the surface and quickly cleaned with a blotting paper. Fruit peels, pulp, and seed (kernel) were removed using a clean sharp knife and homogenized. The fleshes of papaya fruits were dried with lyophilized for further analysis and some of the fresh flesh was used to make juice to evaluate some physicochemical parameters, nutritional composition, and sensory analysis. All the parameters were analyzed using triplicate samples analysis.
Methods of analysis
Color of skin and flesh: Skin and flesh color of papaya fruit were determined through the standard method using a color chart.
Fruit weight: it was determined using a sensitive balance.
Fruit width, length, and diameter: Fruit width, length, and diameter were determined by using a digital caliper.
Juice PH: of the fruit was measured by taking a sufficient quantity of papaya juice sample in 50 ml clean beaker and using PH meter (Type H1 98106 by HANNA).
Total Soluble Solid (TSS): TSS content of the fruit was determined using a pre-calibrated Atago hand refractometer (Type ATAGO, Model-9099). A drop of homogenized papaya pulp was placed at the prism of the refractometer and the lid was closed and TSS read directly from the digital scale at 20°C±1 and results expressed in Brix.
TA (Titerable Acidity): Titerable acidity value was calculated through the standard method (AOAC, 2000) [9]. Zero point zero one molar (0.01M) NaOH was titrated against 10ml of the filtrate using phenolphthalein indicator. The end of the titration was indicated through a change in the color of the sample to pink. Titratable acidity was calculated as follows.
Where 0.01 M = morality of NaOH used, 0.0064= conversion factor for citric acid, since it is major acid in papaya, T = titer value, Ft = quantity of filtrate used, S = quantity of sample weighed and 10 = dilution factor, and 1000 = conversion to mg/100g
Proximate Value; The proximate parameters of ash, crude fat, crude fiber, and protein were determined following with [9] methods.
Carbohydrates (CHO)
It was determined by the difference (the measured protein, fat, ash, and moisture was subtracted from 100 [10],
Gross food energy was estimated by the following equation;
Where FE is the food energy, TC is the total carbohydrate content, CF is the crude fiber, TF is the total fat and CP is the crude protein.
Carotenoids
Carotenoids were performed spectrophotometrically using the method described by [11]. Fresh papaya samples (5g) were ground with cold acetone with a mortar and pestle until the residue became colorless and then vacuum-filtered using a Buchner funnel. The extract was partitioned with petroleum ether, then each fraction was washed with distilled water for complete acetone removal. The extracts were made up to a volume of 50 mL with petroleum ether. All of the procedures were performed in dim light. The extracted carotenoids were collected and measured at 450 nm using a UV spectrophotometer.
Total carotenoids were calculated with the following equation:
Where A is the absorbance, the volume is the total volume of extract (50), A1% 1cm is the absorption coefficient of β-carotene in petroleum ether.
Beta-carotene
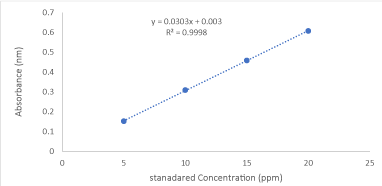
For the extraction of β-carotene, the procedure outlined in AOAC Official Method 941.15- ‘Carotene in Fresh Plant Materials and Silages’ [12]was followed. Fresh papaya samples of 3-gram pulp were weighed and put in mortar and pestle, then with 40 ml acetone, 60 ml petroleum ether, and 0.1 g magnesium carbonate blended for 5 minutes and then extracted until the pulp becomes colorless. Filtration was done with the aid of a suction pump and the sample was decanted into the separator. The residue is washed with two 25 ml portions acetone and then with 25 ml petroleum ether. The extracted samples were evaporated to dryness and the residue was re-dissolved with 5ml of acetone. Then the absorbance of β-carotene is measured at wavelengths of 450 nm. Concentrations of beta-carotene was calculated using standard cultivations curves equations (y=0.0303x + 0.003).
Ascorbic acid/ vitamin C
Vitamin C was determined using a UV-visible spectroscopy method. Precisely, a 5g fresh papaya sample with 100ml of 6% trichloroacetic acid was extracted using mortar and pestle. In the extracted sample 2 drops of saturated bromine solution were added and then 10ml aliquot was taken and mixed with 10ml of 2% thiourea. From the mixture (10 ml extracted and 10ml of 2% thiourea) 4 ml taken into two different test tubes and one as a blank. To each tube, 1ml of 2,4-DNPH was added and put in a water bath at 37 0C for 3 hours and then added slowly 5ml 85% H2SO4 while the tubes are in an ice bath. Added 1ml of 2% DNPH to the blank and mix all tubes and then standing all tubes at room temperature for 30 min. Read the absorbance of the standards, blank and test samples at 515 nm.
Where: As =Absorbance of samples; Ab = Absorbance of blank; A10 µg Std. =The absorbance of 10 µg AA standard
Sensory analysis
The sensory analysis was conducted by a semi-trained panelist of Melkassa agricultural research staff members following the standard procedures for a hedonic scale scoring of 1 to 5. (1- indicates dislike very much and 5- indicates like very much). Samples were evaluated for sweetness, color, flavor, sourness, and overall acceptability by 15 semi-trained panelists.
Statistical analysis
Statistical analysis of the data was carried out using analysis of variance (ANOVA) technique for completely randomized design (CRD)and all pairwise comparisons test was carried out whereas, the Tukey HSD was used for comparison of the treatment means at P< 0.05
Results
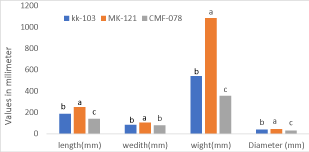
Physical parameters of papaya fruit such as length, width, weight, and diameter were conducted as shown in Figure 2. Among the three-papaya fruit for all parameters of length, width, weight, and diameter, MK-121 variety was the highest on the other hand variety CMF-078 was the lowest for all physical parameters. Fruits of the three papaya varieties showed different physical characteristics such as length, weight, and diameter Knowing of fruit PH, Acidic value is a decisive thing to know fruit characteristics towards spoilage besides its nutritional aspect. In this study fruit PH, TSS, acidic value, skin, and flesh color were included and the result showed that PH value was not significantly different at p<0.05, and its value ranged from 5.284- 5.667. KK-103 papaya variety had a higher TSS value than did the others. Titrable acidity of the three varieties was covered in the range of 1.455- 1.979 as measured by gram/liter. Fruit flesh and skin color were done using a color chart and as a result for variety MK-121 both flesh and skin color (after ripping) was the same while the rest two varieties showed different in their skin and flesh color Table 1.
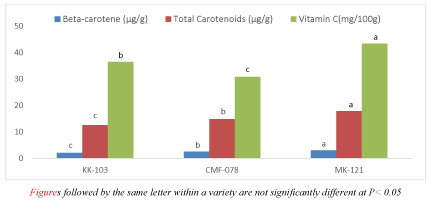
Anti-oxidants such as total carotenoids, beta-carotene, and vitamin C for the three varieties of papaya were conducted. In present finding the total carotenoid, beta-carotene, and vitamin C contents varied from 12.564- 17.860, 2.131- 3.036µg/g and 30.854- 43.407mg/100g respectively Figure 3.
Proximate compositions such as dry matter, ash, protein, fat, fiber, and CHO as well as energy value were analyzed for the newly released papaya varieties and results are presented in Table 2. Values of dry matter, ash, protein, fat, fiber, CHO as well as energy covered 9.809-12.546, 0.411-0.742,0.383-0.719,0.132-0.296,0.758-1.045, 8.851-11.031, and 35.223-45.269 respectively as measured by %.
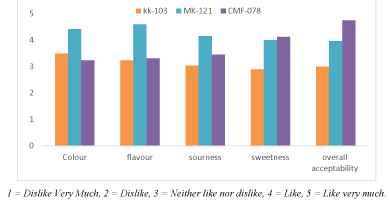
Descriptive sensorial evaluations of papaya fruit were conducted. The sensory analysis carried out using a 5-hedonic scale (where 1 indicates “Dislike Very Much” and 7 represents “Like very much”. Fruit sensory was evaluated for their sweetness, color, flavor, sourness, and overall acceptability by semi-trained panelists and Varieties had shown a difference in their sensorial quality.
Discussion
For the three papaya fruits, physical parameters such as fruit length, weight, diameters, width, skin, and flesh color were conducted and significant variations among the varieties were observed at p≤0.05 for all physical parameters as presented in Figure 2. Results were cover in the range of 138.76 – 250.07mm, 77.870 – 105.38mm, 355.72 – 1082.6 gm, and 29.957 – 44.182 mm as a respective of length, width, weight, and diameter. Among the physical parameters, fruit weight was significantly different for the three papaya varieties followed by their length, width, and diameters. Four varieties of papaya grown at Rajshahi had done for their [8] fruit weight, diameter, and length as a result their values were found in the range of 645.40 - 1740.00 gm, 37- 44 mm, and 190- 250 mm respectively. Therefore, the findings of our research report are in agreement with the above research outputs. Additionally, the above research finding also found that the weight of papaya fruit varied from 486.67g to 1380.33g which is higher than our papaya fruit wight. The physical characteristics of the papaya fruit may vary due to its genotypical variability and also the environmental conditions of the growth area (soil type, geographical locations). On the other hand, the skin and flesh color were the same for variety MK-121 while the rest two varieties of papaya showed different skin and flesh colors as presented in table 1. There are two types of papaya, red-fleshed and yellow-fleshed color as a result of the accumulation of carotenoids in fruit cell chromoplasts. Therefor its color of skin and flesh depending on the accumulations of different carotenoids on their inner parts of the fruit.
Even though the fact that fruit pH for the three varieties was not significantly different at p<0.05, the highest fruit PH obtained from KK-103 and the lowest value found form CMF-078. Total soluble solids (TSS) of the three-papaya fruit varied from 10.287 to 12.620 as measured by Brix and significant variations observed between KK-103 and CMF-078 but KK-103 with MK-121 was not as shown (Table 1). The TSS values of papaya in the present study were similar to those which have been reported by [8,13]. Moreover, [8] also found that between 9.0 to 13.0% for the four varieties of papaya. Among the common acids of fruit and vegetable, citric acid is the one which is the most abundant in papaya fruit and this research output also presented as a citric acid concentration. In this finding fruit acidity (as citric acid) of the three papaya varieties was found to be between 1.454 (MK-121) to 1.978 g/l (CMF-078). Citric acid value for variety CMF-078 was higher than the two papaya varieties and had shown also significant differences at P<0.05. The acidity value of the three-papaya fruits was in agreement with the findings of [13,14]. Fruit with a lower PH and higher an acidity concentration has a better shelf-stable than the others, as a result, it is always chosen by the consumers. Research reports indicated that bioactive compounds, total soluble solids (TSS), and titratable acidity (TA) vary among different papaya cultivars [15,16] due to its differing of growing conditions as well its genotype.
Anti-oxidants
Papaya is a source of antioxidants such as vitamin A, E, B complex, vitamin C as compared to carrots and oranges [17] and also used as the cheapest source of carotenoids, [18]. In this research, the three anti-oxidant of papaya fruit such as vitamin C, total carotenoids, and beta-carotene were analyzed and presented in Figure 4. Varieties of papaya had shown a significant difference for their Vitamin C content at P < 0.05 as shown in Figure 4. The highest vitamin C value was obtained from MK-121 variety (43.407) while the lowest value was found from CMF-078 (30.854). As reported by [8] who fund that the vitamin C content of the four papaya varieties was covered in the range of 41.0 to 42.40mg/100g which is similar finding with one of the papaya varieties. But the other two papaya varieties of our findings were much lower than this report. On the other hand, [19] research report forms five papaw morphotypes the vitamin C content varied from 36.37-43.41 mg/100g which is in agreement with the two papaya fruit results. Papaya fruit has moderate vitamin C sources its content is depending on the variety and ripening conditions [18,20].
Indeed; papaya is one of the crops targeted for nutrient enrichment to be used in sustainable programs to combat vitamin A deficiency in developing nations [21,22] due to high nutrient constituents of carotenoids especially beta- carotene. Beta-carotene, its well knew that used as a precursor to vitamin A which is good for eyesight. Research findings revealed that for both total carotenoid and beta-carotene value, MK-121 variety had shown a significant difference than did other varieties at P < 0.05 with a value of 17.860 µg/g, and 3.036 µg/g whereas variety KK-103 is the lowest 12.564 and 2.131 as shown in Figure 4. Previous research reports of [23,24] found that for different papaya fruit, beta- carotene value obtained in the range of 1.4 - 8.29 ug/g but the total carotenoid content was ranged from 22.88 – 27.03 ug/g. Based on this report, the beta-carotene contents of our papaya fruit are in agreement with the previous findings but the total carotenoid is much lower. Among the variety of papaya, a significant difference at p<0.05 is observed for their vitamin C, total carotenoid, and beta-carotene value. Variability of carotenoids and Vitamins among the varieties of papaya is expected because, it can be influenced by the growing conditions, maturity index, post-harvest handling conditions, as well as variety or cultivar [25].
As revealed and proved in different research reports, the proximate value of fruit and vegetable is not a big deal except for some fruits. The proximate composition of the three papaya fruits included in this research report and varieties had shown a significant difference at p < 0.05 as presented in table 2. For dry matter content, Variety MK-121 followed by CMF-078 showed higher value while KK-103 variety was lowest. Accordingly, [19] research report five papaw morphotypes the dry matter content varied from 8.68-12.53 % which is in line with the present findings. Variety CMF-078 was higher for ash, fat, and fiber contents while KK-103 variety exhibited significantly lower for ash and fat content as presented in Table 2. In ash and fat contents, the three varieties of papaya were significantly different but for protein, variety kk-103 and CMF-078 did not show significant difference while MK-121 was exhibited as a higher at p < 0.05. [19] founds that protein, Ash, fat, crude fiber, and Carbohydrates of the five papaw morphotypes found in the range of 0.47-1.17, 0.31-0.53, 0.37-0.7, 0.83-0.93, and 6.5-9.51 respectively which is in agreement with the current research finding. Carbohydrate is the major nutrient constituent for most fruits and vegetables, the present finding also proves that carbohydrate contents of the papaya varieties are the major constituents of their nutrient. Among the varieties, a significant difference for their carbohydrates and energy values did not show at p < 0.05.
Sensory evaluation
Most of the studies on fresh-cut fruits have been concerned with the objective and subjective evaluation of market quality by color, sensory and texture measurements [26]. In the present study sensory evaluation of the three papaya varieties was performed subjectively and the analysis (color, flavor, sourness, and sweetness) was done for complete ripe fruits. It was carried out by semi-trained panelists using five hedonic scale methods and results presented in Figure 5. The results showed that color, flavor, and sourness values were significantly higher for variety MK-121 than the other two varieties meanwhile variety CMF-078 was higher for sweetness and overall acceptability. According to the panelist evaluation, as a general CMF-078 variety was higher followed by MK-121 for the overall acceptability.
As a general view from this finding, it is evident that the physic-chemical, anti-oxidant and sensorial parameters of papaya varieties differed from one another which are may arise due to different genetic makeup of the variety, climate conditions, growing seasons, site of cultivations and ripening period [27-29]. This list of factors influences the nutrient contents of papaya varieties. Differences in the physic-chemical composition of different papaya varieties are in agreement with the findings of [27,30,31] research report.
Conclusion
Fruit physicochemical parameters of papaya varieties significantly differed from each other, which could probably be due to different genetic make-up of the varieties, bringing about differences in the rate of fruit development. Nevertheless, the results of the present study indicated that fruits of variety CMF-078 exhibited higher overall acceptable value with higher customer preference. On the other hand, variety MK-121 had higher dry matter, protein, carbohydrate content, and energy value than did the other two varieties. It had also a better vitamin C, beta-carotene, and total carotenoids. Therefore, the researcher recommended that fruits of variety MK-121 could be used for fresh consumption as well as for processing purposes to produce vitamin A enriched foods.
This study was carried out with the financial support of the Ethiopian Institute of Agricultural Research (EIAR), Agricultural and Nutrition Research Directorate. The authors are indebted to the Ethiopian Institute of Agricultural Research (EIAR), Melkasa Agricultural Research Center. We would like to thank the National horticultural Research Team of Melkassa Agricultural Research Center for providing quality samples of papaya varieties with detailed information of each variety.
- Anuar NS, Zahari SS, Taib IA, Rahman MT (2008) Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food and Chemical Toxicology 46: 2384-2389. Link: https://bit.ly/3hWlE59
- Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, et al. (2008) The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452: 991-996. Link: https://go.nature.com/2Dvci1i
- Mello VJ, Gomes MTR, Lemos FO, Delfino JL, Andrade SP, et al. (2008) The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomedicine 15: 237-244. Link: https://bit.ly/3jFujt4
- Munoz V, Sauvain M, Bourdy G, Callapa J, Rojas I, et al. (2000) The search for natural bioactive compounds through a multidisciplinary approach in Bolivia. Part II. Antimalarial activity of some plants used by Mosetene indians. Journal of ethnopharmacology 69: 139-155. Link: https://bit.ly/3lOtJLi
- Silva F, Marques A, Chaveiro A (2010) Reactive oxygen species: a double-edged sword in reproduction. The Open Veterinary Science Journal 4. Link: https://bit.ly/3lHzx9H
- Seigler DS, Pauli GF, Nahrstedt A, Leen R (2002) Cyanogenic allosides and glucosides from Passiflora edulis and Carica papaya. Phytochemistry 60: 873-882. Link: https://bit.ly/3gVJJra
- Abbott JA (1999) Quality measurement of fruits and vegetables. Postharvest Biology and Technology 15: 207-225. Link: https://bit.ly/3gOGpOM
- Zaman W, Biswas S, Helali M, Ibrahim M, Hassan P (2006) Physico-chemical composition of four papaya varieties grown at Rajshahi. Journal of Bio-science 14: 83-86. Link: https://bit.ly/3lLOrLV
- AOAC (2000) Official methods of analysis. In: AOAC Gaithersburg, MD. Link: https://bit.ly/3lOPACn
- Pearson D (1976) The chemical analysis of foods: Longman Group Ltd. Link: https://bit.ly/3hYBL1Z
- Rodriguez-Amaya DB, Kimura M (2004) HarvestPlus handbook for carotenoid analysis (Vol. 2): International Food Policy Research Institute (IFPRI) Washington. Link: https://bit.ly/32U2hU0
- Pritwani R, Mathur P (2017) β-carotene content of some commonly consumed vegetables and fruits available in Delhi, India. J Nutr Food Sci 7: 2. Link: https://bit.ly/3554wX9
- Tekliye M (2016) Effect of Storage Duration on Physico-Chemical and Microbiological Quality of Fruit Served in Bahir Dar City, North-West Ethiopia. AJ Chem Pharm Res 4: 49-54. Link: https://bit.ly/3gSImd6
- Zuhair R, Aminah A, Sahilah A, Eqbal D (2013) Antioxidant activity and physicochemical properties changes of papaya (Carica papaya L. cv. Hongkong) during different ripening stage. International Food Research Journal 20: 1653-1659. Link: https://bit.ly/2GrFJSX
- Gomez M, Lajolo F, Cordenunsi B (2002) Evolution of soluble sugars during ripening of papaya fruit and its relation to sweet taste. Journal of Food Science 67: 442-447. Link: https://bit.ly/31TUXs3
- Saeed F, Arshad MU, Pasha I, Na R, et al. (2014) Nutritional and phyto-therapeutic potential of papaya (Carica papaya Linn.): an overview. International Journal of Food Properties 17: 1637-1653. Link: https://bit.ly/3hVXMyn
- Tietze HW (2002) Urine the holy water: Harald Tietze Publishing P/. Link:
- Bari L, Hassan P, Absar N, Haque M, Khuda M, et al. (2006) Nutritional analysis of two local varieties of papaya (Carica papaya L.) at different maturation stages. Pakistan Journal of Biological Sciences 9: 137-140. Link: https://bit.ly/31Vk6me
- Nwofia GE, Ojimelukwe P, Eji C (2012) Chemical composition of leaves, fruit pulp and seeds in some Carica papaya (L) morphotypes. International Journal of Medicinal and Aromatic Plants 2: 200-206. Link: https://bit.ly/2GjG7mk
- Hernández Y, Lobo MG, González M (2006) Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chemistry 96: 654-664. Link: https://bit.ly/31U4i3f
- Organization WH (2003) Diet, nutrition, and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation (Vol. 916): World Health Organization. Link: https://bit.ly/3bnyFSz
- Rodriguez-Amaya DB (2003) Enhancing the carotenoid levels of foods through agriculture and food technology. Internet Paper for Food, Nutrition and Health Theme. Link: https://bit.ly/32RqlH3
- Bhaskarachary K (2008) Papaya carotenoids for combating vitamin A deficiency and age-related macular degenerative diseases. Paper presented at the II International Symposium on Papaya 851.
- Chandrika UG, Jansz ER, Wickramasinghe SN, Warnasuriya ND (2003) Carotenoids in yellow‐and red‐fleshed papaya (Carica papaya L). Journal of the Science of Food and Agriculture 83: 1279-1282. Link: https://bit.ly/3bny7ft
- Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E (1993) Carotenoid content of fruits and vegetables: an evaluation of analytic data. Journal of the American Dietetic Association 93: 284-296. Link: https://bit.ly/31UHFM8
- Ahvenainen R (1996) New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends in Food Science & Technology 7: 179-187. Link: https://bit.ly/352VsSS
- Selvaraj Y, Pal D, Subramanyam M, Iyer C (1982) Changes in the chemical composition of four cultivars of papaya (Carica papaya L.) during growth and development. Journal of Horticultural Science 57: 135-143. Link: https://bit.ly/2GrFcAr
- Souza LMd, Ferreira KS, Chaves JBP, Teixeira SL (2008) L-ascorbic acid, β-carotene and lycopene content in papaya fruits (Carica papaya) with or without physiological skin freckles. Scientia Agricola 65: 246-250. Link: https://bit.ly/32X7xGg
- Wall MM (2006) Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. Journal of Food Composition and Analysis 19: 434-445.
- Brekke JE, Chan HT, Chang TS, Stafford AE (1971) Nonvolatile acids of papaya. Journal of agricultural and food chemistry 19: 263-265. Link: https://bit.ly/2EN1aNY
- Pal D, DK, P, NG D (1980) Studies on the physico-chemical composition of fruits of twelve papaya varieties
- Chonhenchob V, Singh SP (2005) Packaging performance comparison for distribution and export of papaya fruit. Packaging Technology and Science: An International Journal 18: 125-131. Link: https://bit.ly/2Z1XEG5
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
