Journal of Food Science and Nutrition Therapy
Effects of clarified açai (Euterpe oleracea Mart) supplementation on oxidative stress markers in hemodialysis patients: A randomized, controlled pilot study
Isabelle Christine Vieira da Silva Martins1, Denise Mafra2,3, Hervé Rogez4, Maria Conceição Nascimento Pinheiro5, Keuri Eleutério Rodrigues1, Barbarella de Matos Macchi6, Andréa Dias Reis7, Bruna Regis Paiva3, Jessyca Sousa de Brito3, Greicielle Santos da Silva8, Abner Ariel da Silva Lima5, Luís Claúdio Santos Pinto9 and José Luiz Martins Nascimento1,10,11*
2Post-Graduate Program in Cardiovascular Science, Federal University Fluminense (UFF), Niteroi, Brazil
3Post-Graduate Program in Medical Sciences, UFF, Niteroi, Rio de Janeiro, Brazil
4Center for Valorisation of Amazonian Bioactive Compounds (CVACBA), UFPA, Belém, Brazil
5Post- Graduate Program in Tropical Diseases, UFPA, Belém, Brazil
6Biological Sciences Institute, UFPA, Belém, Brazil
7Post-Graduate Program in Movement Sciences, Sao Paulo State University (UNESP), São Paulo, Brazil
8Post-Graduate Program in Nutritional Sciences, UFF, Niteroi, Rio de Janeiro, Brazil
9Post-Graduate Program in Surgery and Experimental Research, Pará State University (UEPA), Belém, Brazil
10National Institute of Science and Technology in Neuroimmunomodulation
11Post-Graduate Program in Pharmaceutical Sciences, Federal University Amapá (UNIFAP), Amapá, Brazil
Cite this as
Da Silva Martins ICV, Mafra D, Rogez H, Nascimento Pinheiro MC, Martins Nascimento JL, et al. (2020) Effects of clarified açai (Euterpe oleracea Mart) supplementation on oxidative stress markers in hemodialysis patients: A randomized, controlled pilot study. J Food Sci Nutr The 6(1): 032-037. DOI: 10.17352/jfsnt.000024Introduction: Patients with Chronic Kidney Disease (CKD) on Hemodialysis (HD) present a reduction of antioxidant enzymes levels and increased production of free radicals. Nutritional strategies have been used to mitigate the this exacerbatedoxidative stress in these patients and supplementation with açai (Amazon fruit Euterpe oleracea) can be a good candidate to exert a protective effect against oxidative stress. The aim of this pilot study was to evaluate the effects of açai supplementation on oxidative stress markers in patients with CKD on HD.
Methods: Eighteen patients were randomized to receive 20 mL of clarified açai pulp juice three times a week for eight weeks (8 patients, 55.5 ± 4.9 years; BMI, 24.8 ± 2.5 Kg/m2) and to be the control group who did not receive any supplementation (10 patients, 56.1 ± 3.4 years, BMI, 25.2 ± 0.7 Kg/m2). Oxidative stress markers plasma levels such as Malondialdehyde (MDA), nitrite, Total Glutathione (TG), Catalase (CAT) and Glutathione Peroxidase (GPx) were evaluated before and after supplementation.
Results: MDA plasma levels were significant reduced from 6.8 ± 1.2 to 5.8 ± 0.9 nmol/mL in açai group (p= 0.037), compared to no significant change in control group. The GSH total level presented slight increase after supplementation in açai group (320.7 ± 59.2 to 492.7 ± 84.8 nmol/mL, p=0.085). For Nitrite, CAT and GPx activity after eight weeks of supplementation with clarified açai (Euterpe oleracea Mart.) pulp there was no change.
Conclusion: The consumption of clarified açai for eight weeks improved lipid peroxidation in HD patients. This result suggests the promising application of açai as an antioxidant food to patients with CKD on HD.
Introduction
Oxidative stress is a very common complication in Chronic Kidney Disease (CKD) patients and becomes more severe in the late stage of the disease, which is exacerbated by the Haemodialysis (HD) procedure [1-3].
Several nutritional strategies have focused on reducing oxidative stress in CKD patients. Among these interventions are the consumption of antioxidants naturally present in some foods whose range of physiological intake is considered safe and can be achieved through food intake [4,5].
Açai (Euterpe oleracea Mart), a typical fruit from the Eastern Amazon basin in Brazil, where is consumed daily by the general population of the northern region of Brazil, has expanded to other regions of the country, especially in recent years for its export to various countries [6,7].
Studies observed that açai fruit has a high antioxidant capacity and is able to bind to free radicals due the range of bioactive compounds, mainly polyphenols, α-tocopherol, anthocyanins and flavones [8-12]. Additionally, these bioactive compounds interact with various enzymes and transcription factors, resulting in protective effects against oxidative stress-induced damage [13-15].
In previous clinical trials with healthy individuals, açai pulp improved lipid profile, lipid peroxidation and free radicals balance, as well as increased levels of antioxidant enzymes [16,17]. Thus, our hypothesis is that açai supplementation in CKD patients may mitigate the oxidative stress, thus leading to clinical improvement. Therefore, the aim of this study is to evaluate the effects of two months of oral açai supplementation on oxidative stress markers in patients with CKD on Hemodialysis (HD).
Material and methods
Participants
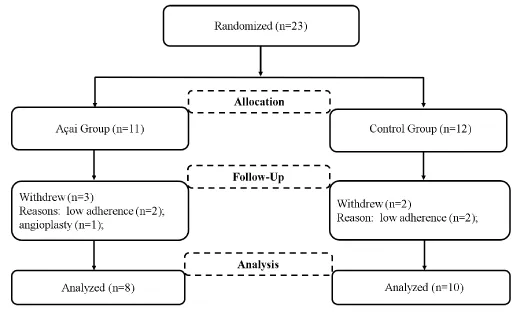
Twenty-three HD patients (açai group: 11 patients and control group: 12 patients) from a hemodialysis Center in Belém, Pará, Brazil were eligible for the randomized controlled trial. Six patients in the açai group and four in the control group withdrew from the study. Therefore, completed the intervention eight patients comprised the açai group and ten the control group (Figure 1).
Patients with access via arteriovenous fistula in an upper extremity; a dialysis duration of 3-4 hours per session three times weekly on a blood flow rate of 350 mL/min to 400 mL/min; and at least a six month history of hemodialysis were included. Patients with autoimmune and infectious diseases, cancer or AIDS; with serum levels of phosphorus and potassium above 5.5 mg/dL; or who used catabolic drugs, antioxidant vitamin supplements or açai were excluded.
The study was performed according to the Ethical Norms for Research with Humans, Resolution 164/96. Approval was granted by the Ethics Committee of Núcleo de Medicina Tropical (No. 2.338.781). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. All patients gave written informed consent to participate in the study and were instructed not to use any supplement during intervention.
Experimental design
The patients were manually allocated with a simple randomization method to the experimental group or the control group by a person who was not involved in the research. Patients received clarified açai (Euterpe oleracea Mart) pulp juice that was lyophilized from 250 mL of açai pulp juice resulting in 20 mL of açai pulp juice diluted in 20 mL of water with the final volume of 40 mL. The açai juice was given orally to the patients after hemodialysis sessions (3x/week) for two months, and the control group did not receive any supplementation. Both groups were advised not to consume açai juice during the period of the research. Measures of biochemical, clinical, anthropometric, oxidative stress data were performed at baseline and after two months.
The routine biochemical parameters, albumin, creatinine, hemoglobin, potassium, phosphorus and Parathyroid Hormone (PTH), were quantified according to standard methods at the hemodialysis center.
Clarified açai (Euterpe oleracea Mart) pulp juice
The samples of the açai pulp juice with fresh fruits were lyophilized and pasteurized. The açai pulp juice were clarified by a patented process licensed (PI 1003060-3). This process consists of the partial purification of the gross aqueous extract produced by the adsorption technique on macroporous resins that resulted an açai pulp juice higher purity antioxidant extract with absence of lipids, proteins and fibers [18,19].
The phytochemical composition to evaluate metabolites with antioxidant activity was performed to measure total polyphenols determined by the Folin-Ciocalteu method and total anthocyanins by a pH differential method [18]. Potassium mineral determination was quantified using an atomic absorption spectrophotometer (Thermo, ICE3000) and expressed in mg/L.
Anthropometric parameters and dietary intake
For the anthropometric evaluation, the body mass index (BMI) was calculated as weight divided by height squared, and then the nutritional status was classified according to the World Health Organization [20].
Food intake was determined by a 24-hour food recall administered by the researchers on three different days – a hemodialysis day, a day without hemodialysis and a weekend day – throughout the experimental period. The quantitative analysis of energy and macronutrient intake were calculated by the software Avanutri & Nutrição, 4.0 version (Três Rios, Brazil).
Collection and separation of blood
The blood was collected by venipuncture in the antecubital region with a vacuum system in tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, before the HD session in earlier in the week. Plasma was separated from blood by centrifugation at 3000 x g for 15 minutes at 4 C, extracted with an automatic pipette, packed in 1.5 mL tubes, and stored at -80°C until analysis.
Determination of thiobarbituric acid-reactive substances (TBARS)
This test indicates estimated by the presence of products derived from lipid peroxidation, mainly malonaldehyde (MDA) using the Ohkawa et al (1979) method [21]. The absorbance was measured in a spectrophotometer at 532 nm (BIO-RAD Model 450 Microplate Reader, Hercules, CA, USA).
Nitrite quantification
Plasma nitrite, final product of nitric oxide (NO), was evaluated by the Griess Reagent method [22]. The samples were analyzed by in a spectrophotometer at 540 nm (BIO-RAD Model 450 Microplate Reader, Hercules, CA, USA).
Total Glutathione (TG)
The detection of total glutathione (GSH and GSSG) [23] was conducted by GSH oxidation by the 5,5-dithio-bis (2-nitrobenzoic acid) reagent, and the GSSG formed was recycled to GSH by glutathione reductase in the presence of NADPH. The absorbance was measured in a spectrophotometer at 412 nm (BIO-RAD Model 450 Microplate Reader, Hercules, CA, USA).
Catalase activity and glutathione peroxidase
The evaluation of catalase (CAT) activity was based on the reaction of the enzyme with methanol in the presence of an optimized concentration of H2O2 [24]. The analysis was performed by in a spectrophotometer at 540 nm (BIO-RAD Model 450 Microplate Reader, Hercules, CA, USA). Glutathione peroxidase (GPx1) activity in serum was determined by kit, was measured indirectly by a coupled reaction with glutathione reductase. The absorbance was measured with a spectrophotometer at 340 nm (Synergy H1M, Winooski, VT, USA).
Statistical analysis
Data were expressed as the mean and Standard Deviation (SD). The normal distribution of the data was verified by the Shapiro-Wilk test. The majority of the variables were normally distributed, except for the kinetic index of dialysis adequacy (Kt/V), parathyroid hormone and CAT activity.
The F test was used on independent parametric variables, and since all tests showed similar variations among the groups, the nonmatched Student’s t-test was applied. The Mann-Whitney test was used for independent nonparametric and ordinal variables, and the chi-squared test was applied to independent and dichotomous variables. The tests were performed using the statistical package Stata with the confidence values set to 95% (p<0.05).
Results
The clarified açai pulp juice offered had total polyphenol content was 1013.2 mg equivalent gallic acid (GAE)/100 mL and total anthocyanins were quantified at 272.1 mg/100 mL, while potassium was quantified at 1174.69 mg/L.
Table 1 shows demographic and biochemical parameters in Açai group (3 males) and Control group (9 males) at baseline moment and Table 2 shows the food intake profile in both groups.
Table 3 shows the effects of supplementation with clarified açai (Euterpe oleracea Mart.) pulp juice on the MDA, nitrite and GSH total level, CAT and GPx activity before and after 2 months in HD patients. The results showed a significant reduction in plasma MDA levels and a slight increase in the total GSH levels after supplementation.
Discussion
During the last decade, açai has been studied because of its richness in bioactive compounds that exert effects on the balance of oxidative stress8. In hemodialysis patients, antioxidant-rich foods seem to have a protective effect on oxidative stress and cardiovascular damage, which is responsible for the high mortality of this group [25-27]. We found with the supplementation of clarified açai pulp juice for two months, a decrease in plasma MDA levels in HD patients, which may have benefits in improving oxidative stress. This study is the first work describing the effects of intervention with clarified açai (ideal model to evaluate the impact of isolated phenolic components and with high antioxidant capacity detected by the DPPH method) in patients with CKD on HD10. Compared to other foods with antioxidant capacity, açai has the highest content of total polyphenols (279.3 mg 100g-1) and antioxidant capacity of 26μM Trolox g-1 than apple, blueberry, raspberry, blackberry, cranberry and strawberry [28-30]. A pilot clinical study randomized, double-blind, placebo-controlled of phase I in healthy individuals, showed that the ingestion of 120 mL juice containing a mixture of fruits and berries including predominantly açai, increased significantly the serum antioxidant capacity at one hour and two hours after consumption as well as reduced the lipid peroxidation [31].
To evaluate oxidative stress, MDA was used as a lipid peroxidation marker, whose reduced plasma levels is associated with progression of CKD stages [32]. Our pioneering study showed, even as a pilot study, results similar to those found with supplementation of red fruit juice with a slightly larger sample size of 21 patients on HD and shorter intervention time for two months [33]. Similar result was found with supplementation pomegranate juice intake in 101 HD patients for one year [34]. In animal model of renovascular hypertension, the extract of açai around 200 mg/kg, was also able to prevent lipid peroxidation [35]. The mechanism involved appears to be related to the anthocyanins that have an important role on protection in the cells of oxidative damage, this probably involves its action as a reduction in ROS produced in polymorphonuclear cells, and reduced of migration of pro-inflammatories [31]. However, it is still uncertain if lipid peroxidation may not only be the cause, but also the result of cell damage, since progression of CKD stages is associated with the value of MDA and increased consumption of antioxidant enzymes [36,37].
Furthermore, it is inaccurate to assume a dose-time effect due to the low bioavailability of anthocyanins in organisms and the associated absorption factors that lead to synergistic açai components and metabolite effects because they induce intestinal microbiota modification [38].
Our work suggests that supplementation with açai may be beneficial in protecting against damage caused by oxidative stress. Moreover, quantifying açai recommendations is still needed because dietary and pharmacological interaction factors are unknown [38].
As limiting factor of this work, the small size of the sample, the absence of the evaluation of the antioxidant capacity and the total phenolic compounds plasma levels. However, this was the first randomized pilot trial that obtained time-response association between clarified açai juice and reduction in lipid peroxidation in HD patients.
Conclusion
In conclusion, despite our study having a small sample size, the consumption of clarified açai for eight weeks it seems improved lipid peroxidation in HD patients. This result indicates the promising application of açai as an antioxidant action in patients with CKD. Thus, it is desirable outcomes that polyphenol-rich interventions may inhibit or slow down oxidative stress in hemodialysis patients. From this finding, future studies in larger populations are needed to verify how much the açai is able to assist in combating the sequelae of free radicals.
- Lakshmi BS, Harini BD, Suchitra MMP, Rao PVLNS, Kumara VS (2018) Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren Fail 40: 534-540. Link: https://bit.ly/35dNHIa
- Guo CH, Chen PC, Hsu GS, Wang CL (2013) Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients 5: 1456-1470. Link: https://bit.ly/2FIGOWq
- Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A (2016) Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol 27: 354-370. Link: https://bit.ly/35cyres
- Biesalski HK, Dragsted LO, Elmadfa I, Grossklaus R, Müller M, et al. (2009) Bioactive compounds: definition and assessment of activit.Nutrition 25: 1202-1205. Link: https://bit.ly/3jmOMT7
- Peake JM, Gobe GC, Fassett RG, Coombes JS (2011) The effects of dietary fish oil on inflammation, fibrosis and oxidative stress associated with obstructive renal injury in rats. Mol Nutr Food Res 55: 400-410. Link: https://bit.ly/2Tc7YYN
- Rogez H (2002) Açai: Preparo, Composição e Melhoramento da Conservação. 2002. 1st ed. Universidade Federal do Pará, Brazil.
- PARÁ. SEFA (2015) Documento mimeografado. 2015. Belém, PA, Brazil.
- Heinrich M, Dhanji T, Casselman I (2011) Açai (Euterpe oleracea Mart.) — A phytochemical and pharmacological assessment of the species’ health claims. Phytochem Lett 4: 10-21. Link: https://bit.ly/31qpehx
- Arrifano GFP, Lichtenstein MP, Souza-Monteiro JR, Farina M, Rogéz H, et al. (2018) Clarified Açaí (Euterpe oleracea) Juice as an Anticonvulsant Agent: In Vitro Mechanistic Study of GABAergic Targets. Oxid Med Cell Longev 20: 1-6. Link: https://bit.ly/31jAjkz
- Souza-Monteiro JR, Hamoy M, Santana-Coelho D, Arrifano GP, Paraense RS, et al. (2015) Anticonvulsant properties of Euterpe oleracea in mice. Neurochem Int 90: 20-27. Link: https://bit.ly/2Hi5qFV
- Petruk G, Illiano A, Del Giudice R, Raiola A, Amoresano A, et al. (2017) Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. J Photochem Photobiol 172: 42-51. Link: https://bit.ly/34dDbRZ
- Poulose SM, Bielinski DF, Carey A, Schauss AG, Shukitt-Hale B (2017) Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with Açai-enriched diets. Nutr Neurosci 20: 305-315. Link: https://bit.ly/34dKgSj
- Ferrari D, Speciale A, Mariateresa C, Fratantonio D, Molonia MS, et al. (2016) Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol Lett 5: 51-58. Link: https://bit.ly/3dFe11H
- Thilavech T, Abeywardena MY, Adams M, Dallimore J, Adisakwattana S (2017) Naturally occurring anthocyanin cyanidin-3-rutinoside possesses inherent vasorelaxant actions and prevents methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed. Biomed Pharmacother 95: 1251-1259. Link: https://bit.ly/2FIIhvU
- Choi YJ, Kim N, Nam RH, Lee S Lee HS, et al. (2017) Açai Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice. Gut Liver 11: 243-252. Link: https://bit.ly/2Tb1JEE
- Sadowska-Krępa E, Kłapcińska B, Podgórski T, Szade B, Tyl K, et al. (2015) Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defense capacity and lipid profile in junior hurdlers. A pilot study. Biol Sport 32: 161-168. Link: https://bit.ly/3o2mEIp
- Barbosa PO, Pala D, Silva CT, de Souza MO, do Amaral JF, et al. (2016) Açai (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 32: 674-680. Link: https://bit.ly/2Tgofff
- Rogez H, Moura FG, Larondelle Y (2017) Kinetic modeling of anthocyanin degradation and microorganism growth during postharvest storage of açai fruits (Euterpe oleracea). J Food Sci 77: C1300-C130. Link: https://bit.ly/2Tb1U2M
- Pompeu DR, Rogez H, Monteiro KM, Tinti SV, Carvalho JEV (2012) Capacidade antioxidante e triagem farmacológica de extratos brutos de folhas de Byrsonima crassifolia e de Inga edulis. Acta Amaz 42: 165-172. Link: https://bit.ly/3o7Z64L
- World Health Organization (2000) Obesity: preventing and managing the global epidemic: report of a WHO Consultation. Geneva, Switzerland: WHO Technical Report Series 894. Link: https://bit.ly/2Tgokj3
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358. Link: https://bit.ly/3oavtQv
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126: 131-138. Link: https://bit.ly/3ketPuP
- Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159-3165. Link: https://bit.ly/2T6Px7W
- Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity Anal Biochem 184: 193-199. Link: https://bit.ly/3kkOFZy
- Jun M, Zhu B, Tonelli M, Jardine MJ, Patel A, et al. (2012) Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 60: 2061-2071. Link: https://bit.ly/34fL5u6
- Dahwa R, Fassett RG, Wang Z, Briskey D, Mallard AR, et al. (2014) Variability of oxidative stress biomarkers in hemodialysis patients. Biomarkers 19: 154-158. Link: https://bit.ly/35dLKeF
- Esgalhado M, Stenvinkel P, Mafra D (2017) Nonpharmacologic Strategies to Modulate Nuclear Factor Erythroid 2-related Factor 2 Pathway in Chronic Kidney Disease. J Ren Nutr 27: 282-291. Link: https://bit.ly/2Hj4aCG
- Pacheco-Palencia A, Hawken P, Talcott T (2007) Phytochemical, antioxidant and pigment stability of açai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res Int 40: 620-628. Link: https://bit.ly/2Host24
- Pereira ACS, Wurlitzer NJ, Dionisio AP, Lacerda Soares MV, Rocha Bastos Mdo S, et al. (2015) Synergistic, additive and antagonistic effects of fruit mixtures on total antioxidant capacities and bioactive compounds in tropical fruit juices. Arch Latinoam Nutr 65: 119-127. Link: https://bit.ly/31lC8O0
- Wang SY, Lin HS (2000) Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem 48: 140-146. Link: https://bit.ly/3kdWwbf
- Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, et al. (2008) In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem 56: 8326-8333. Link: https://bit.ly/2Hj01Pb
- Xu G, Luo K, Liu H, Huang H, Fang X, et al. (2015) The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren Fail 37: 45-49. Link: https://bit.ly/358bZTC
- Spormann TM, Albert FW, Rath T, Dietrich H, Will F, et al. (2008) Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiol Biomarkers Prev 17: 3372-3380. Link: https://bit.ly/2HhCm1t
- Shema-didi L, Sela S, Ore L, Shapiro G, Geron R, et al. (2012) One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med 53: 297-304. Link: https://bit.ly/35dQUaG
- Costa CA de, Ognibene DT, Cordeiro VSC, de Bem GF, Santos IB, et al. (2017) Effect of Euterpe oleracea Mart. Seeds Extract on Chronic Ischemic Renal Injury in Renovascular Hypertensive Rats. J Med Food 20: 1002-1010. Link: https://bit.ly/2T9wjhZ
- Toborek M, Wasik T, Drózdz M, Klin M, Magner-Wróbel K, et al. (1992) Effect of hemodialysis on lipid peroxidation and antioxidant system in patients with chronic renal failure. Metabolism 41: 1229-1232. Link: https://bit.ly/35g75E9
- Xu G, Luo K, Liu H, Huang T, Fang X, et al. (2015) The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren Fail 37: 45-49. Link: https://bit.ly/3lYiEGM
- Fernandes I, Faria A, Calhau C, Freitas V. de Mateus N (2014) Bioavailability of anthocyanins and derivatives. J Funct Foods 7: 54-66. Link: https://bit.ly/3jcr2AP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
